Canine Cushing’s syndrome
Written by Bradley Bishop and Patty Lathan
Canine Cushing’s syndrome (Canine hyperadrenocorticism or HAC) is one of the more frequently encountered endocrinopathies in dogs, characterized by clinical signs of cortisol excess; the most common presenting signs are polyuria and polydipsia.
Article
Key Points
Naturally occurring hyperadrenocorticism is a result of excess cortisol secretion, which is caused by either an adrenal or pituitary tumor. Physical examination and history findings are key to making a diagnosis.
There are a variety of screening and differentiating tests for hyperadrenocorticism, and the clinician needs to exercise judgment in both the choice of test and interpretation of the results.
Treatment is not recommended in patients if there are no clinical signs.
Optimal treatment for patients with adrenal tumors is adrenalectomy, whereas medical treatment is recommended in patients with pituitary-dependent disease.
Introduction
HAC can be caused by either an adrenal tumor (AT, 15% of cases) or a pituitary tumor, resulting in pituitary-dependent hyperadrenocorticism (PDH, 85% of cases). Iatrogenic hyperadrenocorticism, caused by excessive administration of glucocorticoids, is also possible. Adrenal tumors directly secrete excess cortisol, whereas with pituitary tumors excess adrenocorticotropic hormone (ACTH) is released, stimulating the adrenal cortex to secrete excess cortisol. Most cases of PDH are caused by a microadenoma, a tumor so small that it does not cause neurological signs. However, macroadenomas can also occur, and these may eventually lead to neurological disease.
Clinical presentation
A thorough history and diligent examination are essential to begin the process of diagnosis. There are many clinical signs that may indicate a patient has HAC – and whilst a dog does not need to have all of the presenting complaints to be diagnosed, the more abnormalities present, the more likely it is that HAC is the correct diagnosis. It is also important to bear in mind that atypical presentations can occur. Patients with HAC are not usually “sick” – so if a dog presents with vomiting, diarrhea, or anorexia, HAC is unlikely to be the primary diagnosis, and any evaluation for it should be delayed until the other disease is identified and treated.
History and physical examination
The median age at presentation is between 10-12 years, and while any breed can be affected, small breed dogs are especially predisposed to PDH [2]. However, approximately half of adrenocortical tumors (AT) are found in dogs weighing more than 20 kilograms. Females are slightly more likely to develop both PDH and AT than males [3].
The most common complaint voiced by owners is polyuria and polydipsia (PU/PD) [3],[4]; this is because cortisol decreases release of antidiuretic hormone (ADH) from the pituitary, inhibits ADH activity in the kidney, and causes psychogenic polydipsia. Polyphagia is also common, but constant begging to go outside or inappropriate urination are often the clinical signs that drive owners to take their dog to the clinic.
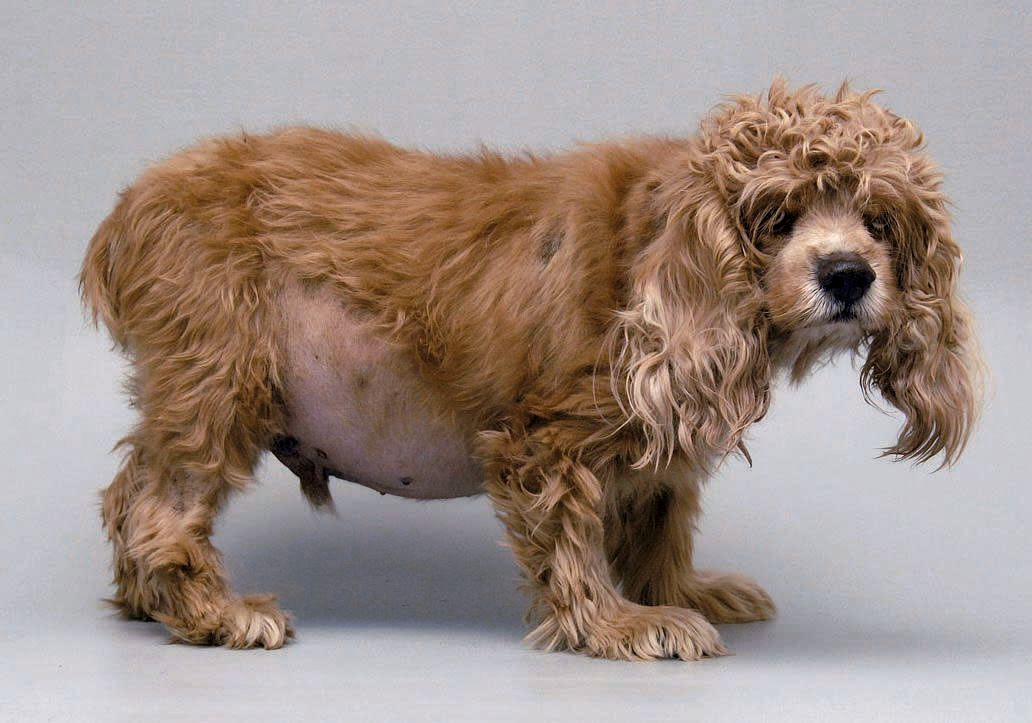
A pot-bellied appearance is common in dogs with HAC. Whilst affected animals are almost always polyphagic, the increase in abdominal size is rarely due to weight gain. Rather, hepatomegaly and weakening of the abdominal musculature from the catabolic effect of excess cortisol results in the pot-bellied appearance. Skin lesions are an extremely common finding; physical examination often reveals a bilaterally symmetric alopecia, sometimes only sparing the head and distal extremities. Other dermatologic signs include thin skin, hyperpigmentation, comedones, pyoderma, and calcinosis cutis.
Table 1 summarizes the typical clinical signs, while Figure 1 depicts a Cocker Spaniel with the classical appearance of the disease.
Table 1. Initial history and physical examination findings in dogs presenting with HAC.
| Most common | Less common |
|---|---|
|
Polyuria
Polydipsia
Polyphagia
Abdominal distension
Bilaterally symmetric alopecia
Pyoderma
Hepatomegaly
Weakness
|
Thin skin
Calcinosis cutis
Hyperpigmentation
Comedones
Lethargy
Testicular atrophy
Thromboembolism
Ligament rupture
|

Laboratory testing
Prior to performing any HAC screening tests, routine laboratory diagnostics should be undertaken on any animal with suspicious clinical signs. In addition to providing evidence for HAC, these tests will help rule out other differential diagnoses and concurrent conditions. HAC screening tests should not be performed without significant suspicion from a combination of history, clinical signs and laboratory testing. No single abnormality from complete blood count, serum chemistry, or urinalysis is pathognomonic for the disease, but certain results may serve as an indication for further testing [5]; common laboratory abnormalities are summarized in Table 2.
Table 2. Common laboratory findings in patients with HAC.
| Complete blood count | Serum chemistry | Urinalysis |
|---|---|---|
|
Neutrophilia
Monocytosis
Lymphopenia
Eosinopenia
Thrombocytosis
Mild erythrocytosis
|
Increased ALP
Increased ALT
Hyperglycemia (mild)
Hypercholesterolemia
Decreased BUN
|
Specific gravity < 1.020
Proteinuria
Urinary tract infection*
|
*often requires culture for diagnosis
Complete blood count
Due to excessive production of cortisol, a stress leukogram (neutrophilia, monocytosis, lymphopenia, and eosinopenia) is often observed in affected animals. Mild thrombocytosis and polycythemia are occasionally present [1],[5].
Serum chemistry
The most commonly increased value in dogs with HAC is the enzyme alkaline phosphatase (ALP), which is elevated in approximately 90% of cases. An increase in ALP is a sensitive indicator of HAC, but is not specific due to the presence of numerous isoenzymes of ALP (glucocorticoid-induced, hepatic, bone, placental, intestinal). Even though elevated ALP is common, as yet there is no evidence that the degree of elevation correlates with the likelihood of HAC being present, therefore an extreme increase in ALP is no more indicative of the disease than a mild increase. In addition, alanine amino-transferase (ALT) is frequently elevated due to swollen hepatocytes, accumulation of glycogen, or disruption of hepatic blood flow as a result of steroid hepatopathy [1].
Glucocorticoids result in hyperglycemia via two mechanisms: increased hepatic gluconeogenesis and insulin antagonization. However, the increase is generally mild (< 150 mg/dL or 8.3 mmol/L) and concurrent diabetes mellitus is uncommon (5% of HAC cases). Serum cholesterol concentrations are increased in the majority of HAC dogs, as a result of glucocorticoid-stimulated lipolysis.
Blood urea nitrogen (BUN) is frequently decreased, as the diuresis related to PU/PD causes constant urinary loss of BUN and medullary washout.
Urinalysis
Since most affected dogs have PU/PD, urine specific gravity is usually < 1.020. Proteinuria is common, but rarely severe enough to cause hypoalbuminemia or hypoproteinemia. If the proteinuria is severe (urine protein:creatinine ratio > 2-3), another cause of protein-losing nephropathy should be suspected.
Due to immunosuppression from persistently high serum cortisol levels, urine culture should be performed on all suspected cases; approximately 50% of dogs with HAC have a urinary tract infection (UTI) at the time of examination [6]. Since cortisol is anti-inflammatory, and the urine is dilute, an active sediment is not always present in HAC dogs with UTI’s, so a urine culture should always be performed in affected dogs – and indeed as part of the workup for all PU/PD cases.
Diagnostic imaging
Diagnostic imaging is not mandatory for diagnosis and treatment of HAC, although it often helps to differentiate PDH from AT. However, given that most affected dogs are geriatric, abdominal and thoracic imaging may also help identify concurrent conditions such as neoplasia that should be addressed prior to treatment of hyperadrenocorticism.
Radiography
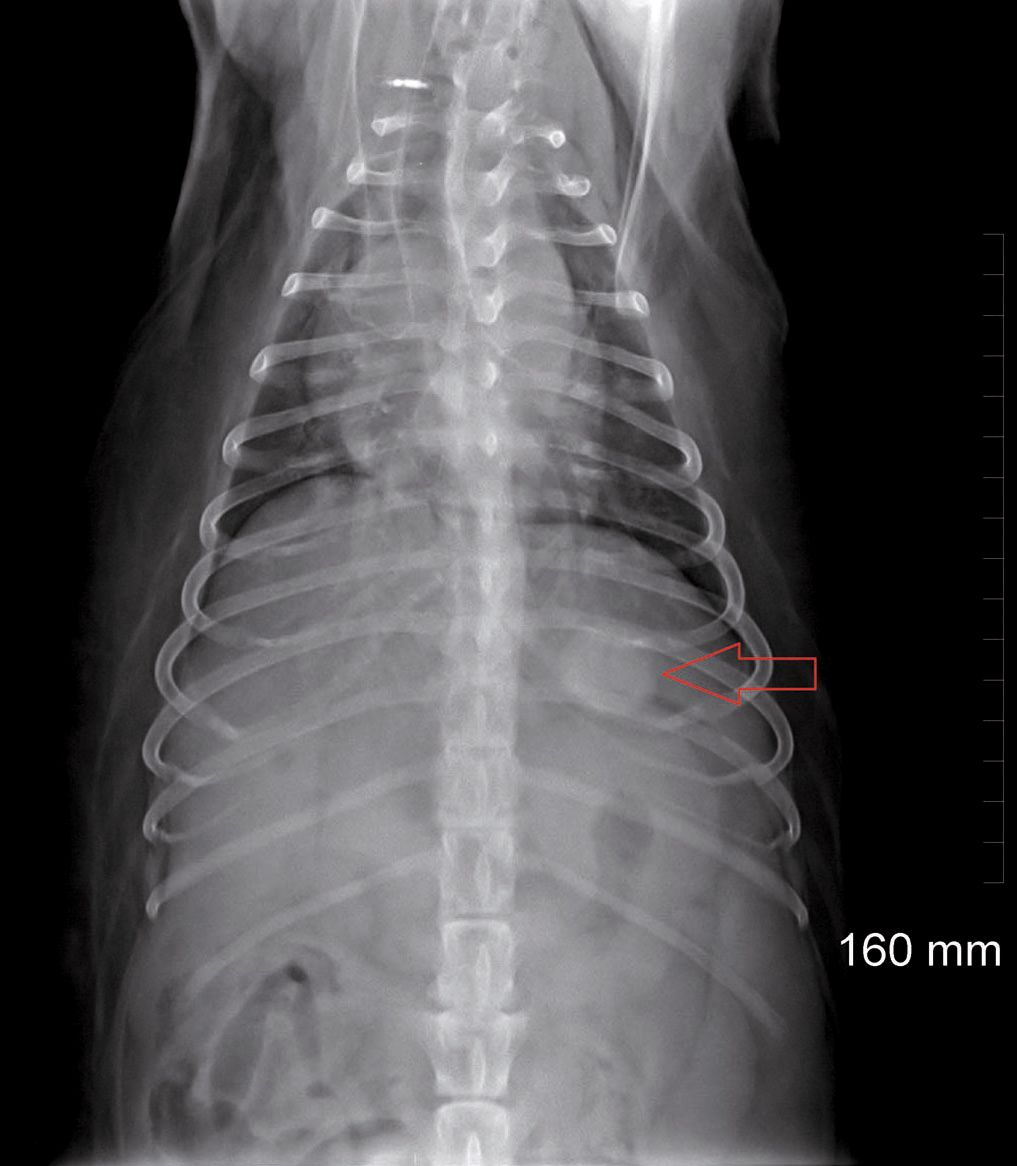
The most common radiographic change associated with HAC is hepatomegaly [7]. In some dogs with adrenal tumors, unilateral adrenomegaly with mineralization is identified, although the presence of mineralization does not help differentiate between adenoma and carcinoma. If there is calcinosis cutis, peripheral soft tissue mineralization may be appreciated. Thoracic radiographs may reveal bronchial and tracheal mineralization, or pulmonary metastases from an adrenocortical carcinoma (Figure 2). Approximately 50% of adrenal tumors are carcinomas, and of these approximately 50% will have metastasized by the time of diagnosis [3].
Abdominal ultrasonography
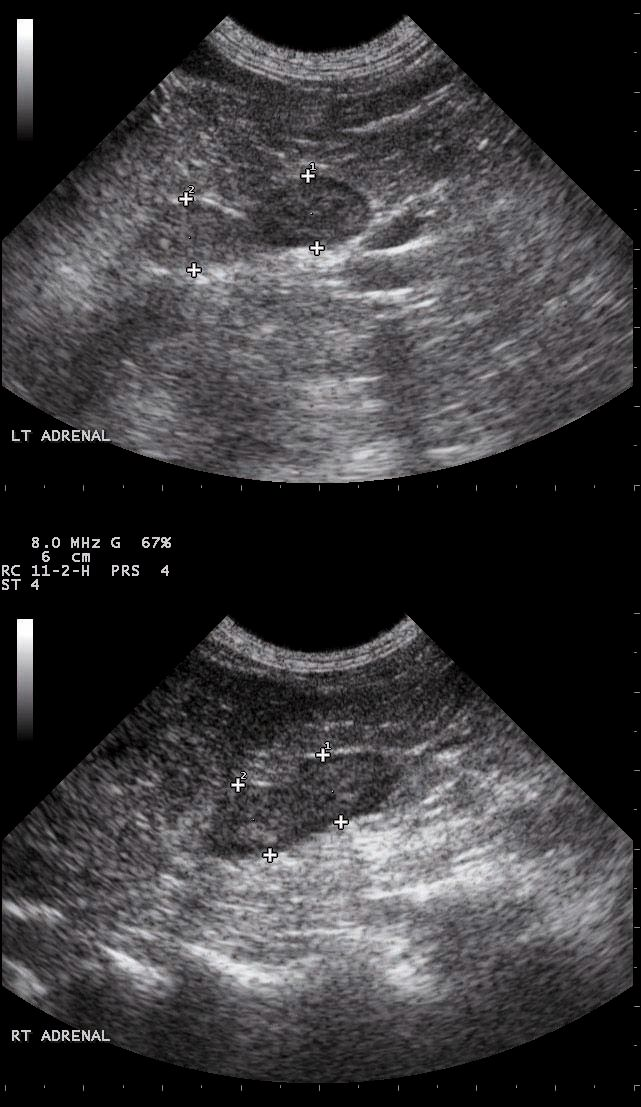
Ultrasonography is useful for assessing the adrenal glands and liver as well as identifying any concurrent disease. Evaluation of the size and shape of the adrenal glands may help differentiate between AT and PDH. Adrenal glands in PDH are usually bilaterally enlarged (> 6-7 mm diameter), but with a relatively normal shape (Figure 3). However, PDH cannot be ruled out if the adrenal glands are not enlarged. If an adrenocortical tumor is present, one adrenal is often enlarged and irregularly shaped with a small, atrophied contralateral gland due to decreased circulating ACTH concentration.
Advanced imaging
Computed tomography (CT) and magnetic resonance imaging (MRI) are both sufficient for the identification of a pituitary macroadenoma (classically defi as a pituitary mass > 10 mm, but more recently as a mass that can be seen with the naked eye), so the modality selected should be based on cost and availability. Advanced imaging of the pituitary is recommended in dogs diagnosed with PDH. In those animals that are already neurologically affected, imaging can be used to confirm the presence of a macroadenoma, whilst for those that have no neurological signs, a scan may either detect the presence of a macroadenoma or help determine if one may develop in the future. Studies have shown that approximately 10-25% of PDH patients develop neurologic signs within a year of diagnosis of HAC [8], and that signs are most likely to develop if the pituitary mass is > 10 mm, so radiotherapy is recommended to help shrink pituitary macroadenomas if they are found to be > 8 mm in size. However, in a patient without neurological signs, brain imaging is not recommended unless the owners anticipate requesting radiation therapy should a large tumor be identified [8].
Advanced abdominal imaging is much more sensitive for diagnosis of AT than radiography. If surgical removal of an adrenal gland is indicated, CT or MRI is extremely useful in localizing the tumor and assessing its invasiveness, permitting development of a surgical plan prior to celiotomy.
Diagnostic tests
Since the disease can be caused by either a pituitary or an adrenal tumor, both screening and differentiating tests are recommended. The screening test should be performed first, before conducting further investigations to differentiate between PDH and AT (since the prognosis and recommended treatment will vary) once the diagnosis of HAC is confirmed.
Screening tests
Low-dose dexamethasone suppression test (LDDST)
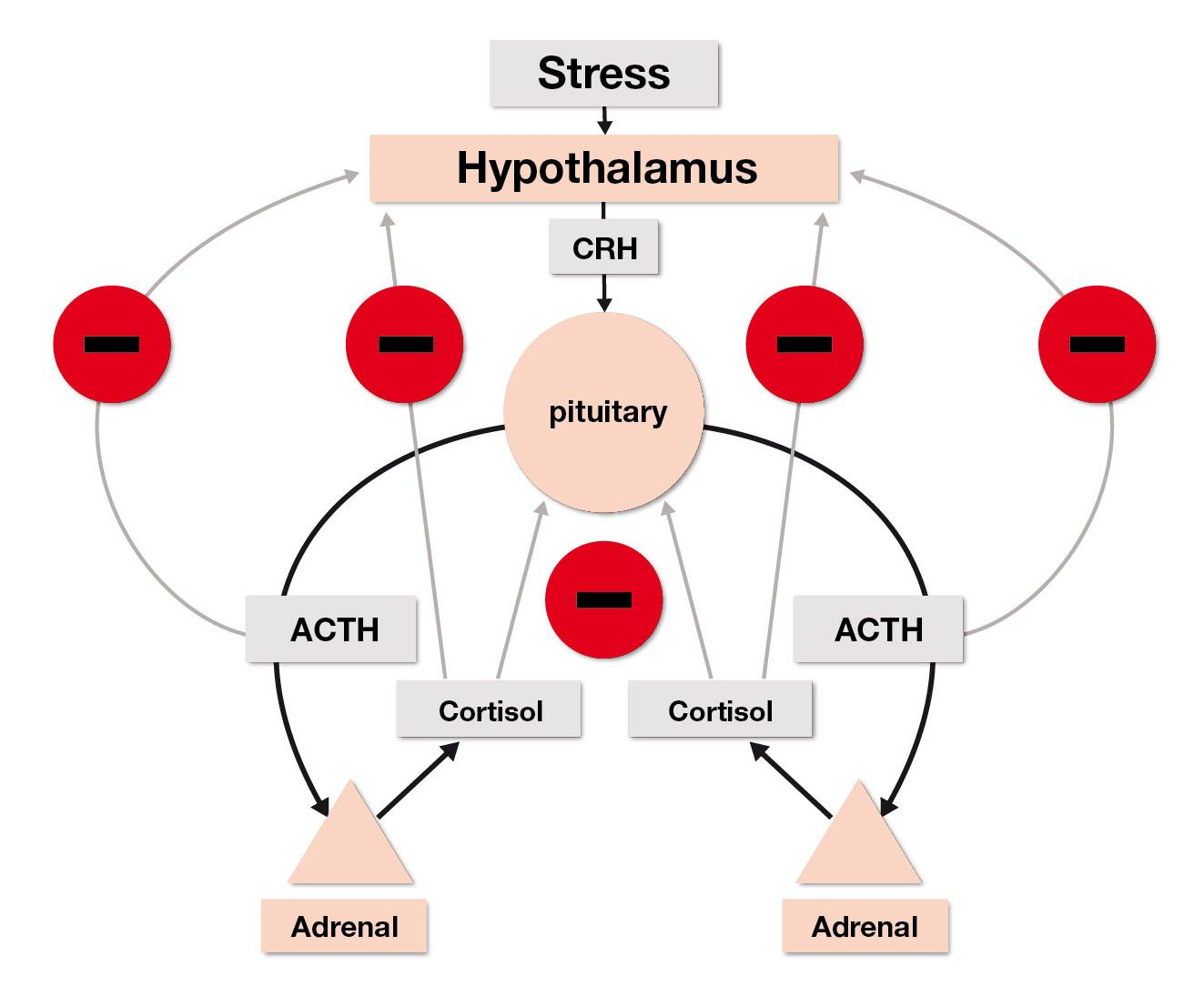
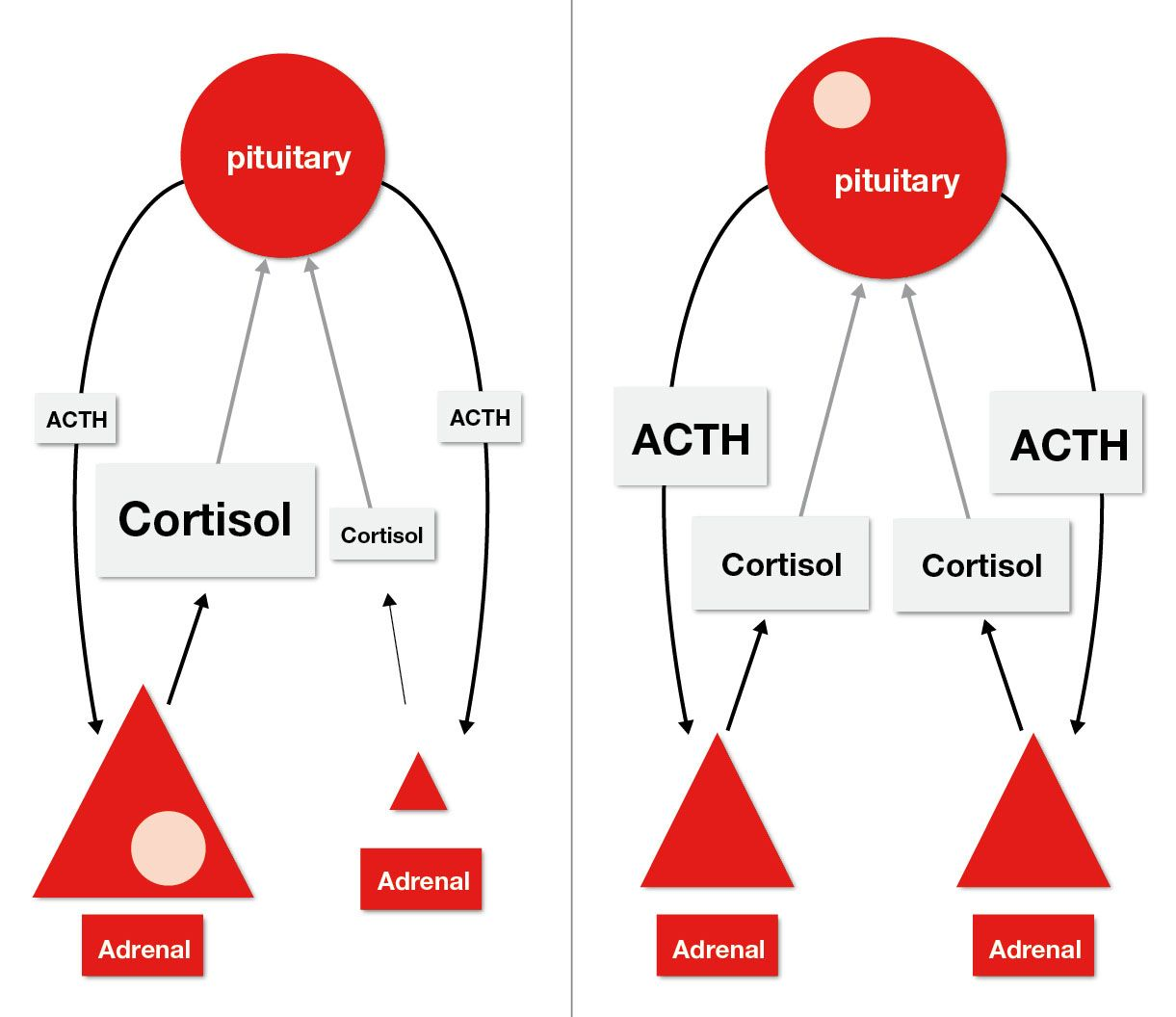
The LDDST is used to demonstrate decreased sensitivity of the hypothalamic-pituitary-adrenal axis (HPAA) to negative glucocorticoid feedback [5]. The normal HPAA is shown in Figure 4, whilst Figure 5 displays the differences between the HPAA of dogs with adrenal tumors and pituitary tumors. In a healthy dog dexamethasone administration will cause suppression of pituitary release of ACTH, resulting in lower plasma cortisol concentration 8 hours later. However, in patients with PDH or AT, the cortisol concentrations will not be adequately suppressed, due to the autonomic production of ACTH and cortisol, respectively. Dexamethasone is used because it does not interfere with the cortisol assay.
To perform the test a serum sample is drawn prior to administration of 0.01 mg/kg dexamethasone IV to determine the dog’s baseline cortisol concentration; repeat blood samples are taken at 4- and 8-hours post-administration and submitted for analysis of cortisol concentration. A diagnosis of HAC is made by examining the level at the 8-hour interval. Due to the spectrum of disease and difference between patients, no specific cut-off point can diagnose every patient, but a cortisol concentration of greater than 1.4 µg/dL (39 nmol/L) at 8 hours post-dexamethasone is commonly seen as failure of suppression and indicative of HAC.
In addition to serving as a screening test, in certain circumstances the LDDST can differentiate between PDH and AT. Once HAC is confirmed by inadequate suppression at 8 hours, further examination of the cortisol concentrations at 4 and 8 hours may be performed. Three different parameters may be used to diagnose PDH using the LDDST: cortisol concentrations less than 50% of baseline at 4 hours, cortisol concentrations less than 50% of baseline at 8 hours, or cortisol concentrations less than 1.4 µg/dL (39 nmol/L) at 4 hours post-dexamethasone administration. Lack of suppression does not allow for differentiation, and further testing must be performed to make a definitive diagnosis.
Sensitivity of the LDDST is excellent and has been reported to be between 85-100% [5]. However, the specificity of the test can be low (44-73%) due to stress or if there is concurrent illness, and because of this it should not be performed prior to addressing concurrent diseases. Even with the low specificity, the LDDST is considered the screening test of choice for canine HAC.
ACTH stimulation test
The ACTH stimulation test uses exogenous synthetic ACTH (cosyntropin or tetracosactrin) to test adrenal reserve [5]. Due to the increased adrenocortical mass in dogs with HAC, they have the capacity to secrete excessive quantities of cortisol. Sensitivity of ACTH stimulation testing ranges from 57-95%, with higher sensitivity for PDH cases. The specificity is higher (59-93%) than seen with the LDDST. A baseline serum cortisol concentration is obtained prior to IV or IM administration of 5 µg/kg (up to 250 µg/dog) synthetic ACTH. One hour following administration, another serum cortisol concentration should be evaluated. As previously stated, dogs with HAC often produce excessive amounts of cortisol following the administration of ACTH due to the increased adrenocortical mass, so levels of 17-22 µg/dL (470-607 nmol/L) are considered a “gray area” for HAC diagnosis, while concentrations > 22 µg/dL (607 nmol/L) are considered diagnostic. Glucocorticoid, progestagen, and ketoconazole administration are all known to suppress cortisol concentrations, and can result in false negative results. Due to the lower sensitivity of the ACTH stimulation test, a patient with a post-ACTH cortisol concentration less than 17 µg/dL, but with clinical signs consistent with HAC, should be tested using a LDDST prior to ruling out the disease.
Urine corticoid:creatinine ratio (UCCR)
Excretion of creatinine is relatively stable, so the UCCR adjusts for changing concentrations of blood and accurately reflects cortisol production in the absence of kidney disease [5]. A free-catch urine sample is obtained and the ratio of cortisol vs. creatinine is determined; note that the sample should be obtained from the first urination of the day and for 2 to 3 consecutive days, averaging the results; a ratio of less than 15-20 is considered negative for HAC. The test is extremely sensitive (75-100%), but has a very low specificity (20-25%) when the sample is obtained in the veterinary hospital, due to increased cortisol secretion from the stress of transport and hospitalization. Owner collection of urine at home at least two days after a visit to a veterinarian is suggested. Due to low specificity, UCCR should primarily be used to rule out the likelihood of HAC, rather than to aid in its diagnosis.
Differentiating tests
High-dose dexamethasone suppression test (HDDST)
Dogs with PDH that do not exhibit cortisol suppression with the LDDST may exhibit suppression following the HDDST [5]. This test is performed using 0.1 mg/kg of dexamethasone IV, and otherwise follows the same protocol as the LDDST. Cortisol suppression is defined as serum cortisol levels below the reference range (usually 1.4 µg/dL or 39 nmol/L) at 4 or 8 hours, or serum concentrations less than 50% of baseline at 4 or 8 hours. Whereas dogs with ATs rarely suppress following either LDDST or HDDST, approximately 65% of dogs with PDH show signs of cortisol suppression following the LDDST, and 75% suppress following the HDDST. Given this minimal increase in differentiation versus the LDDST, the HDDST is only recommended in cases where endogenous canine ACTH (eACTH) and abdominal ultrasound are not available.
Endogenous ACTH concentration
eACTH is secreted in an episodic manner in normal dogs and in those animals with PDH. eACTH should be below the reference range in dogs with AT, due to the negative feedback of cortisol on the pituitary gland [5]. However, dogs with PDH do not have a properly functioning pituitary; as the gland is resistant to negative feedback, this usually results in normal to high eACTH concentrations. However, due to episodic secretion, eACTH concentrations in dogs with PDH may be below the limit of detection of some assays.
The biggest problem with eACTH testing is that proper handling of the sample is of the utmost importance; failure to follow protocol may result in inaccurate readings. Blood should be instantly transferred to a chilled, silicon-coated plastic tube containing EDTA upon collection. The sample should then be centrifuged within 15 minutes and the plasma immediately decanted into a plastic tube and frozen. The plasma must stay frozen until it is analyzed, so appropriate care and precautions should be considered for shipment. Alternatively, addition of aprotinin prevents ACTH degradation by plasma proteases, but may cause false decreases in readings when used with certain assays. Consultation with the laboratory for specific handling instructions is recommended prior to sample collection.
Treatment
There are several options available for treatment of HAC. However, even if the disease is present in a dog, treatment is not recommended if no clinical signs are present. The treatment method selected is dependent upon a variety of factors such as lesion location (PDH or AT), owner finances, and veterinarian preference.
Surgical therapy
Adrenalectomy is the treatment of choice for small, non-invasive adrenal tumors. Dogs with AT have a good long-term prognosis following successful surgery, but intra and peri-operative mortality is approximately 20-30% [9],[10]. Computed tomography is recommended to help determine if there is extensive invasion of the surrounding vasculature and tissues [3]. Following unilateral adrenalectomy, the patient must be supplemented with a tapering dose of glucocorticoids so that the atrophied contralateral adrenal gland can have time to respond to ACTH and return to normal function.
Trans-sphenoidal hypophysectomy is an effective surgical option available for PDH, but unfortunately there are few locations where this surgery is performed and it requires significant specialty training. A remission rate of 91% after one year and 80% after two years has been reported [11].
Medical therapy
Medical therapy is recommended for PDH, and also for adrenal tumors in which patient or client factors preclude adrenalectomy. The two most common medications used in veterinary medicine are trilostane and mitotane (o,p’-DDD), although availability and product license varies between countries. Studies have failed to show significant differences between the efficacies of these drugs in treating both AT and PDH, and selection of medication is frequently dependent upon veterinarian experience and preference. In the authors’ experience the use of trilostane has a shorter learning curve and is more straightforward than mitotane.
Trilostane, which in many countries is currently the only approved drug for treatment of both PDH and AT in dogs, is a competitive inhibitor of 3β-hydroxysteroid dehydrogenase. This inhibition decreases the synthesis of cortisol, aldosterone, and androstenedione in the adrenal cortex, although the decrease in cortisol synthesis is most significant.
Trilostane should be given with food, as this significantly increases its gastrointestinal absorption. Its duration of activity is between 10-18 hours, which means that cortisol synthesis will increase as the drug is metabolized; however, clinical signs may or may not return before the next dose is administered. Published protocols for the use of trilostane vary. The authors’ preference is to start with a single daily dosing at 2-3 mg/kg in the morning, changing to twice-daily dosing if a dog shows clinical signs (e.g., PU/PD) in the evening, although other authors recommend starting with twice-daily dosing. Between 10-14 days after commencing treatment serum biochemistry and an ACTH stimulation test should be performed to determine the efficacy of the current dose, and since the test must be started 3-5 hours following administration of the trilostane, morning administration is optimal.
Once treatment has commenced, Table 3 shows the recommended course of action based on post-ACTH serum cortisol levels and clinical signs. Note that the effect of trilostane appears to increase throughout the first month, so the dose is not usually increased at the first recheck unless the post-stimulation cortisol is > 10 µg/dL (275 nmol/L). Following this first recheck, the protocol may be closely followed, with dosage usually adjusted by 10-25% each time as appropriate. If the dog’s poststimulation cortisol is < 2 µg/dL (55 nmol/L) and the dog does not show clinical signs of illness or Addisonian crisis, the trilostane may be stopped; if clinical signs reappear, the drug can be re-started at a lower dose.
Table 3. Trilostane therapy actions after acth stimulation test.
| Serum cortisol concentration | Appropriate action |
|---|---|
| < 2 µg/dL (55 nmol/L), signs of hypocortisolemia | Treat as Addisonian; do not start trilostane again until an ACTH stimulation test confirms recovery |
| < 2 µg/dL (55 nmol/L), no clinical signs of illness | Stop therapy until clinical signs recur and begin with lower dose |
| 2-6 µg/dL (55-165 nmol/L) | Continue current therapy |
| 6-9 µg/dL (165-248 nmol/L) | If no clinical signs of HAC are present, continue with current therapy. Increase dose if patient is showing clinical signs |
| > 9 µg/dL (248 nmol/L) | Increase dose |
If there are signs of hypocortisolemia (vomiting, diarrhea, decreased appetite, etc.), trilostane should be discontinued, and if the dog becomes severely unwell and/or has hyponatremia and/or hyperkalemia, it may need hospitalization for treatment as an Addisonian crisis. Alternatively, if the signs are mild, the dog may be discharged with oral dexamethasone (0.1-0.2 mg/kg q24H). Trilostane therapy should not be re-instituted (at a 10-25% decreased dose) until clinical signs of HAC recur and an ACTH stimulation test demonstrates adequate adrenal reserve.
Following the first recheck, dogs should be rechecked at 14 days, then 30 days, and every 3 months thereafter. During these rechecks, serum chemistry should also be evaluated to assess the electrolytes. Since HAC is a clinical disease, it is necessary to perform ACTH stimulation tests at these intervals to practice optimal medicine, but if a client has limited financial resources and reports that the dog is doing well clinically, a single baseline cortisol may be performed to screen for hypoadrenocorticism, although this will usually result in inferior control of the disease. If the baseline cortisol levels are greater than 2 µg/dL (55 nmol/L) and there are no adverse clinical signs, trilostane therapy may be continued. However, if the baseline is less than this, an ACTH stimulation is required prior to increasing the trilostane dose.
Aside from clinical signs associated with cortisol deficiency, adverse effects are uncommon following trilostane administration. Lethargy and inappetence during the first few days of treatment are sometimes seen. Mild serum chemistry abnormalities (hyperkalemia and azotemia) have been reported. However, idiosyncratic adrenal necrosis occurs in some dogs, an unpredictable response that may occur at any time during treatment with no known cause. These patients will have cortisol deficiency with or without electrolyte abnormalities, and usually need emergency therapy as for a hypoadrenocortical crisis. Although rare, the owner must be warned about the risk so that they know what to look for. Notably, in the authors’ experience, if a dog experiences a full Addisonian crisis with electrolyte abnormalities whilst on trilostane, the dog is likely to remain Addisonian for life.
Caution should be exercised when using trilostane in conjunction with angiotensin converting enzyme inhibitors due to the aldosterone-lowering effects of both medications. Mild hyperkalemia (< 7 mmol/L) is not uncommon, but more severe hyperkalemia requires medication adjustment.
Trilostane is commercially available in a variety of capsule concentrations, but very low doses (e.g., 5 mg per day) are occasionally necessary in very small dogs. Compounding trilostane is complicated and commercial pharmacies may use the unapproved base chemical rather than the licensed drug. At least one study documented significant variation in drug content and absorption characteristics when trilostane was prepared from an unapproved source [12], so it is essential to ask a pharmacy to utilize the approved product if compounding the drug.
Mitotane was previously the most commonly prescribed medication for treatment of HAC. The drug causes selective necrosis of the zona fasciculata and zona reticularis of the adrenal cortex, and usually spares the zona glomerulosa (except in cases of overly sensitive patients and inadequate monitoring), so electrolyte concentrations are usually normal in these dogs. Treatment consists of two phases: induction and maintenance. During the induction phase, high dosages of mitotane are given daily for 7-10 days, until any decrease in clinical signs or onset of adverse effects (such as anorexia, lethargy, vomiting, etc.) is observed, and the ACTH stimulation test shows adequate control. A weekly dosage is then given as maintenance, in an effort to prevent the cells destroyed during the induction phase from growing back. Potential side effects include signs of hypoadrenocorticism and liver toxicity.
Trilostane and mitotane are by far the most commonly used drugs for treatment of HAC, but l-deprenyl and ketoconazole have been used in the past. L-deprenyl is a dopamine agonist that works by providing irreversible inhibition of monoamine oxidase type B. The effects of the drug are on the pars intermedia of the pituitary gland, which is the location for around 30% of pituitary tumors causing PDH. The drug is extremely well-tolerated with few side effects, but only a small percentage of dogs show a response to treatment, therefore its use is not recommended for PDH. Ketoconazole is an imidazole that inhibits 11β-hydroxylase and therefore has the ability to inhibit steroidogenesis. Following administration, some dogs experience lowered levels of circulating cortisol, but it is not as consistently effective as mitotane and trilostane, and is not currently recommended for the treatment of HAC where mitotane and/or trilostane are available [13].
Conclusion
Canine hyperadrenocorticism is a common endocrinopathy, but there is currently no single test that allows definitive diagnosis. Treatment can be either medical or surgical, although again there is no one preferred option. Since most cases are due to pituitary tumors, medical treatment is the most common option, although regular monitoring of clinical signs and assessment using blood testing is imperative, as over-treatment can be potentially fatal. However, with proper monitoring and client compliance, dogs can achieve a good quality of life while being treated for hyperadrenocorticism.
Bradley Bishop
BSc, Mississippi State University College of Veterinary Medicine, Starkville, Mississippi, USA
United States
Bradley Bishop graduated from Mississippi State University with a Bachelor of Science in Biological Sciences in 2011 and is due to graduate from Mississippi State University College of Veterinary Medicine this year. At present he is performing clinical rotations and advanced electives during his final year of veterinary school. His primary area of interest is small animal endocrinology.
Patty Lathan
VMD, MS, Dip. ACVIM, Mississippi State University College of Veterinary Medicine, Starkville, Mississippi, USA
United States
Patty Lathan is an Associate Professor of Small Animal Internal Medicine at the Mississippi State University College of Veterinary Medicine. She attended college at Texas A&M University and veterinary school at the University of Pennsylvania, then completed an internship at Mississippi State University before finishing a residency in small animal internal medicine at Purdue University. Her primary interest is endocrine disease.
References
- Feldman EC, Nelson RW. Canine hyperadrenocorticism (Cushings Syndrome). In: Canine and Feline Endocrinology and Reproduction. St. Louis, Missouri: Saunders, 2004;252-352.
- Peterson, ME. Diagnosis of hyperadrenocorticism in dogs. Clin. Tech. Small Anim. Pract. 2007;22(1):2-11.
- Reusch CE, Feldman EC. Canine hyperadrenocorticism due to adrenocortical neoplasia: pretreatment evaluation of 41 dogs. J. Vet. Int. Med. 1991;5(1):3-10.
- Ling GV, Stabenfeldt GH, Comer KM, et al. Canine hyperadrenocorticism: pretreatment clinical and laboratory evaluation of 117 cases. J. Am. Vet. Med. Assoc. 1979;174(11):1211-1215.
- Behrend EN, Kooistra HS, Nelson R, et al. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J. Am. Vet. Med. Assoc. 2013;27(6):1292-1304.
- Forrester SD, Troy GC, Dalton MN, et al. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. J. Vet. Int. Med. 1999;13(6):557-560.
- Huntley K, Frazer J, Gibbs C, et al. The radiological features of canine Cushing’s syndrome: a review of forty-eight cases. J. Small Anim. Pract. 1982;23(7):369-380.
- Bertoy EH, Feldman EC, Nelson RW, et al. One-year follow-up evaluation of magnetic resonance imaging of the brain in dogs with pituitary-dependent hyperadrenocorticism. J. Am. Vet. Med. Assoc. 1996;208(8):1268-1273.
- Schwartz P, Kovak JR, Koprowski A, et al. Evaluation of prognostic factors in the surgical treatment of adrenal gland tumors in dogs: 41 cases (1999-2005). J. Am. Vet. Med. Assoc. 2008;232(1):77-84.
- Barthez PY, Marks SL, Woo J, et al. Pheochromocytoma in dogs: 61 cases (1984-1995). J. Vet. Int. Med. 1997;11(5):272-278.
- Meij B, Voorhout G, Rijnberk A. Progress in trans-sphenoidal hypophysectomy for treatment of pituitary-dependent hyperadrenocorticism in dogs and cats. Mol. Cell Endocrinol. 2002;197(1-2):89-96.
- Cook AK, Nieuwoudt CD, Longhofer SL. Pharmaceutical evaluation of compounded trilostane products. J. Am. Anim. Hosp. Assoc. 2012;48(4):228-233.
- Peterson ME. Medical treatment of canine pituitary dependent hyperadrenocorticism (Cushing’s disease). Vet. Clin. North Am. (Small Anim. Pract.) 2001;31(5):11.
Other articles in this issue
Share on social media