Diagnosis and treatment of canine chronic hepatitis
Written by Cynthia RL Webster
Chronic hepatitis is a common disease in dogs but can often go undetected, especially in the early stages; Cynthia Webster presents an overview of the disease, with an emphasis on the diagnostic and treatment options.
Key Points
An accurate diagnosis of chronic hepatitis (CH) requires pathologic evaluation of multiple hepatic biopsies – preferably obtained by laparoscopy – from different liver lobes.
All liver biopsy specimens should be evaluated for quantitative copper concentration, since excess hepatic copper is an important and treatable cause of CH.
Significant hepatic inflammation can be present in dogs that have no clinical or diagnostic imaging signs of liver disease.
In dogs with idiopathic CH where a meticulous search for other causes has been undertaken, a trial of immunosuppressive medication is indicated to determine if immune-mediated hepatitis is present.
Introduction
| • Moderate-to-marked portal, lobular or centrilobular lymphocytic, plasmacytic and/or granulomatous inflammation • Interface hepatitis (inflammation that breaches the limiting plate and spills over into the lobule) • Varying degrees of hepatocyte cell death (apoptosis or necrosis) • +/- Bile duct proliferation • +/- Fibrosis • +/- Nodular regeneration |
Box 1. Key histopathologic features of chronic hepatitis.
Chronic hepatitis (CH) can occur in any breed of dog, and the onset can be insidious. It progresses to end stage cirrhosis when significant fibrosis and nodular regeneration develops, and is defined histopathologically by certain key features, as outlined in (Box 1) [1]. It is critical to differentiate CH from a histologic diagnosis of non-specific reactive hepatitis, where mild-to-moderate inflammatory infiltrates in the portal, lobular and centrilobular regions are present without evidence of cell death or degeneration. These infiltrates are due to the escape of inflammatory cytokines and endotoxins from disease elsewhere in the splanchnic bed [2].
Etiology
In the majority of dogs with CH the etiology cannot be determined, so-called idiopathic CH [3] [4], but various causes or potential causes are worthy of note.
Several studies have failed to identify hepatotropic viruses in dogs with CH, but histopathological and/or serologic evidence of Leptospira bacteria have been identified in laboratory colonies of dogs, and more recently molecular means have identified leptospiral organisms in dogs with granulomatous hepatitis [5]. Whether it is the organism or an immune reaction to the organism which causes the CH is unknown. Leishmaniasis is associated with granulomatous CH, whilst other bacterial (Bartonella), rickettsial (Ehrlichia, Anaplasma) and protozoal (Neospora, Toxoplasma, Sarcocystis) infections may cause canine CH. These infections, however, are more often acute to subacute, and part of a more systemic disease process.
Several drugs and supplements have the potential to cause CH in the dog, and clinicians should be vigilant in obtaining a complete medication history [6]. Most drugs can potentially cause acute liver injury, but a few, including anticonvulsants (phenobarbital, primidone and phenytoin), oxibendazole, lomustine (CCNU), amiodarone, mitotane and NSAIDs can lead to chronic hepatic inflammation.
Copper toxicity is also a potential etiology. Normally, many dogs consume excess copper (Cu) in their diet. Copper entering the liver must bind to Cu-binding proteins or be excreted in the bile, since free Cu causes oxidative stress leading to hepatocellular death. Normal hepatic Cu concentrations in the dog are 120-400 µg/g dry weight (DW) [7]. Hepatic damage (as evidenced by increased serum alanine aminotransferase (ALT) activity and morphologic changes) begins when levels exceed 1000 µg/g DW, and damage is invariably present with values of 1500 µg/g DW or higher [7] [8] [9]. There is, however, considerable phenotypic variability in an individual dog’s response to excess Cu. Some dogs have toxic hepatic Cu levels but no evidence of liver damage, while others have only mild elevations in Cu and severe damage [9] [10] [11]. Although any breed of dog can accumulate copper, several breeds exhibit a predilection (Table 1) [7]. In some dogs, e.g., the Bedlington Terrier, copper accumulation is due to genetic aberrations in copper-handling proteins. Accumulating evidence, however, suggests that dietary copper excess also contributes to the increasing incidence of copper-associated CH (Cu-CH) reported in the last two decades [10] [11]. Some 20 years ago many petfood companies switched dietary supplementation from copper oxide (which has a very poor bioavailability) to the more bioavailable copper chelates. This change, coupled with the fact that the US National Research Council has not established a maximum limit for dietary copper, has resulted in some commercial diets containing excess amounts of highly bioavailable copper [12] [13]. In Europe FEDIAF1 has established a maximum value for copper concentrations in canine diets, although studies suggest that dogs, particularly those with breed predilections, may accumulate hepatic copper when fed diets with copper concentrations below this level [14] [15]. Several studies have now demonstrated that dogs (both with and without CH) over the last two decades have higher hepatic copper concentrations compared to similar populations of dogs pre-1998 [10] [11]. A diagnosis of Cu-CH requires evaluation of a hepatic biopsy specimen which will show the presence of CH accompanied by rhodamine-positive copper accumulation, primarily in centrilobular hepatocytes, and elevated hepatic copper levels (> 400 µg/g DW, typically greater than 1000 µg/g DW). There are, however, several challenges in making the diagnosis of Cu-CH. These include lobe-to-lobe variability in copper concentration, the presence of significant fibrosis which can decrease copper levels, the challenge that regenerative nodules lack copper accumulation, and the fact that later-stage inflammatory/fibrotic changes complicate determination of lobular distribution.
| Breed | Etiology | Genetic basis |
|---|---|---|
| Bedlington Terrier | Copper | Yes, COMMD1 (majority) or ABCB12 |
| Dalmatian | Copper | Yes, but no gene identified |
| Labrador Retriever |
Copper (1/3 of cases) Idiopathic/immune |
Yes; ATP7B in about 1/3 dogs |
| Doberman Pinscher |
Copper Immune |
Unknown |
| English and American Cocker Spaniel | Idiopathic/immune | Unknown |
| English Springer Spaniel | Idiopathic/immune | Unknown |
| West Highland White Terrier | Copper Idiopathic |
Yes, but no gene identified |
Table 1. Breed predispositions to chronic hepatitis.
1 Fédération européenne de l'industrie des aliments pour animaux familiers
A diagnosis of immune-mediated CH is often considered when no other etiology has been identified. Although specific criteria for such a diagnosis have not been developed, an immune basis in dogs with idiopathic CH is suggested by the presence of a moderate-to-marked lymphocytic infiltrate on histopathology, positive serum auto-antibodies, a familial history of CH, an association with other autoimmune diseases (e.g., hypothyroidism, atopy, inflammatory bowel disease), gender (females are generally more likely to be affected) and a favorable response to immunosuppressive therapy [13]. A presumptive clinical diagnosis of immune-mediated CH requires the meticulous elimination of other potential etiologies (infectious, environmental or foodborne toxins, drugs).
Signalment and clinical signs

Chronic hepatitis can occur in any dog including crossbreeds, but several breed predilections exist (Table 1) [16]. Chronic hepatitis is generally a disease of middle-aged dogs, but cases have been reported as young as 5 months and as old as 17 years. There is a female predisposition in Labradors, Dobermans, Dalmatians and English Springer Spaniels, and a male predisposition in Cocker Spaniels (Figure 1).
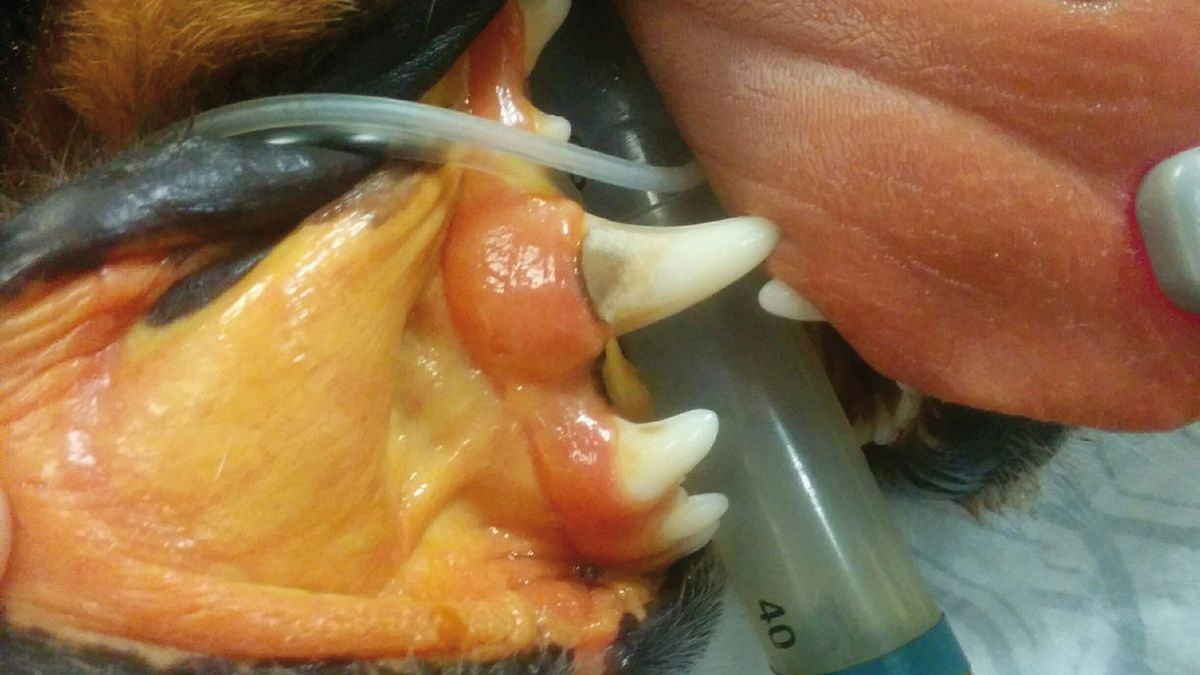
The most common clinical signs in affected dogs are non-specific and may include lethargy/depression and anorexia. Polyuria and polydipsia (PU/PD) are two of the earliest signs. More specific signs of liver disease, such as jaundice, hepatic encephalopathy and ascites, are less common and typically indicate the presence of advanced disease (Figure 2a) (Figure 2b) (Figure 2c).
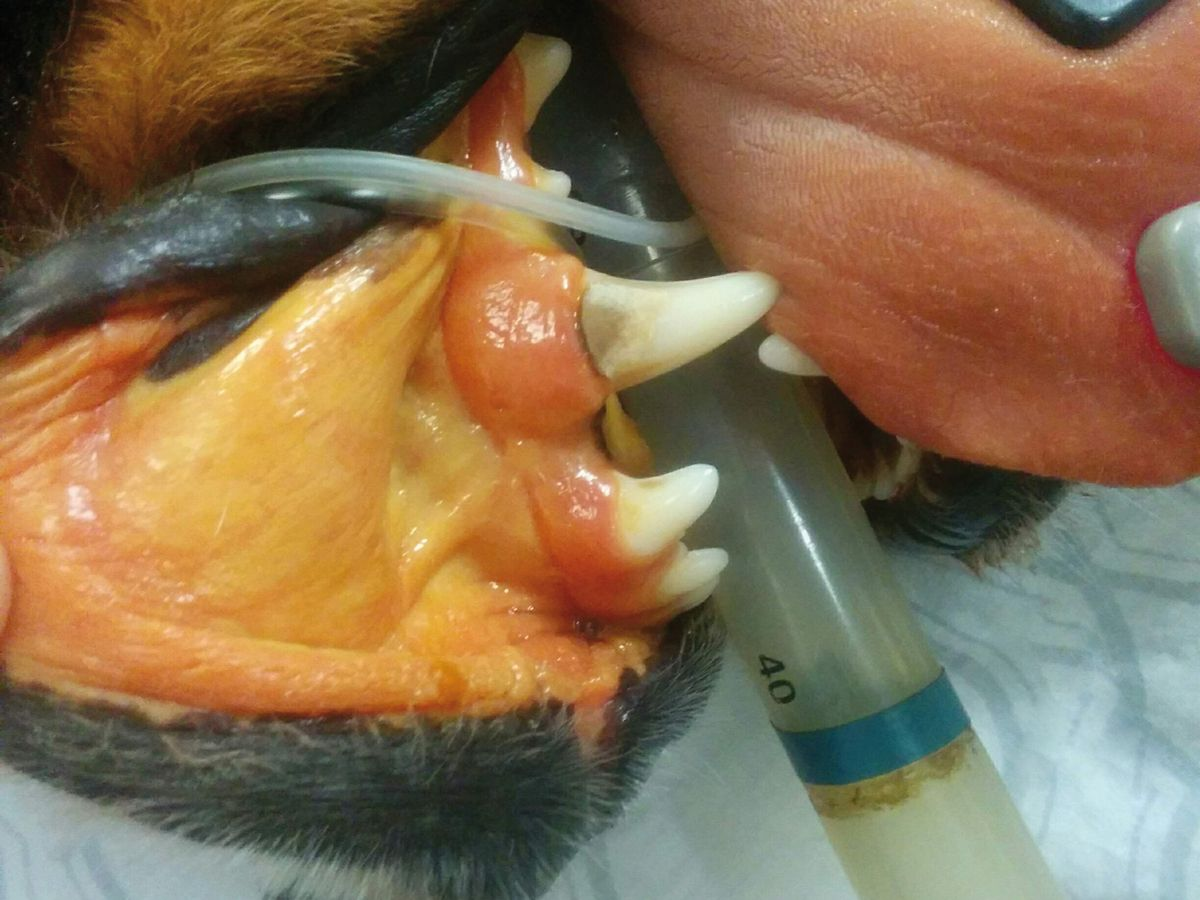
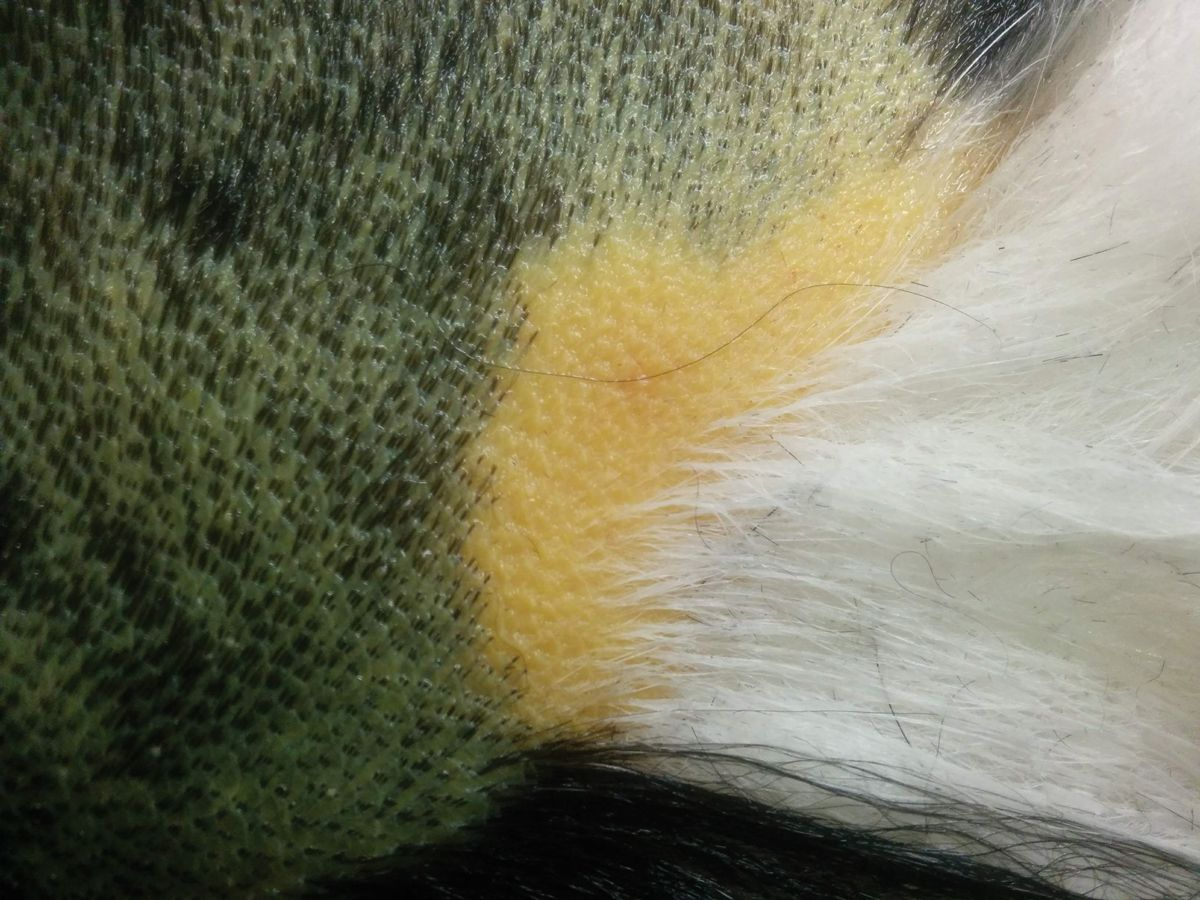
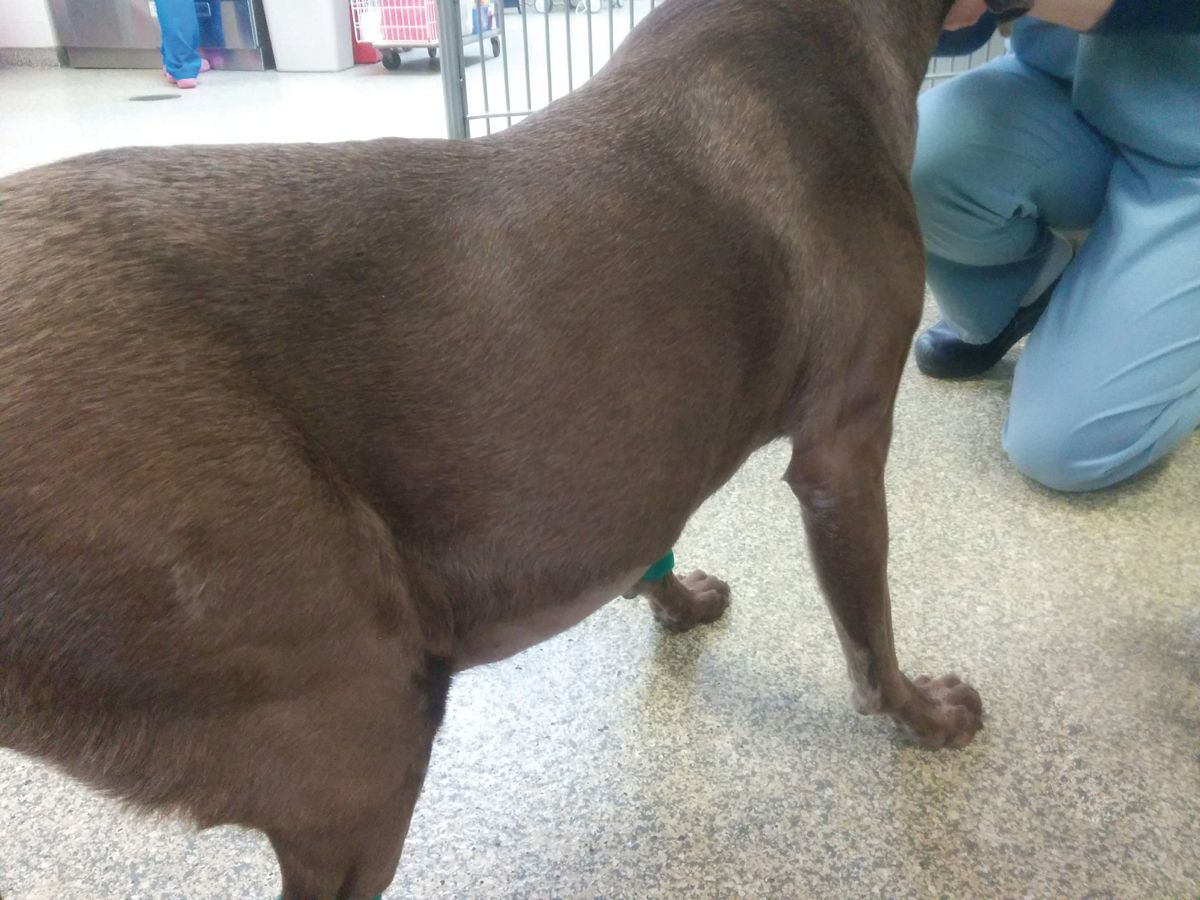
Because of the reserve capacity of the liver, many dogs with CH are subclinical, with the disease picked up on routine blood screens that show increased serum liver enzyme activity. It is at this stage that diagnosis should be pursued, as therapeutic intervention in advanced disease is often less successful.
Clinical pathology
Serum alanine aminotransferase (ALT) is the best screening test for CH, although its sensitivity is only around 70-80%. Significant histological lesions can therefore exist in the absence of accompanying ALT elevation. The magnitude of the ALT elevation activity is typically greater than that seen for serum alkaline phosphatase (ALP) activity, and elevations in ALP occur later in the disease. In late-stage cirrhosis, serum levels of liver enzymes may fall markedly as hepatocytes are replaced by fibrous tissue. The frequency of other clinical pathologic signs is summarized in (Table 2).
| Parameter | % of dogs with change | # studies (# dogs) |
|---|---|---|
| Increased ALT | 85 +/-15 | 10 (250) |
| Increased ALP | 82 +/-18 | 10 (250) |
| Increased ASP | 78 +/-10 | 3 (56) |
| Increased GGT | 61 +/-12 | 5 (121) |
| Decreased BUN | 40 +/-29 | 5 (65) |
| Hypoalbuminemia | 49 +/-19 | 15 (323) |
| Hypocholesterolemia | 40 +/-12 | 4 (118) |
Table 2. Common biochemical changes in dogs with chronic hepatitis.
Total serum bile acids (TSBA) are not a screening test for CH. Using 20-25 mmol/L as a cut-off, the sensitivity for pre-and post-prandial bile acids to detect CH is only about 50%. Since bile acids are very sensitive to shunting of blood around the liver, the sensitivity for cirrhosis when portal hypertension and multiple acquired portosystemic shunts (MAPSS) exist increases to almost 100%. It is ill-advised to wait until TSBA are elevated before proceeding to hepatic biopsy, as by this point significant and perhaps irreversible hepatic changes have occurred.
As PU/PD is a common clinical sign in CH, accompanying isosthenuria is seen on urinalysis. A transient acquired Fanconi syndrome (glucosuria with normoglycemia) is associated with Cu-CH [7].
Imaging
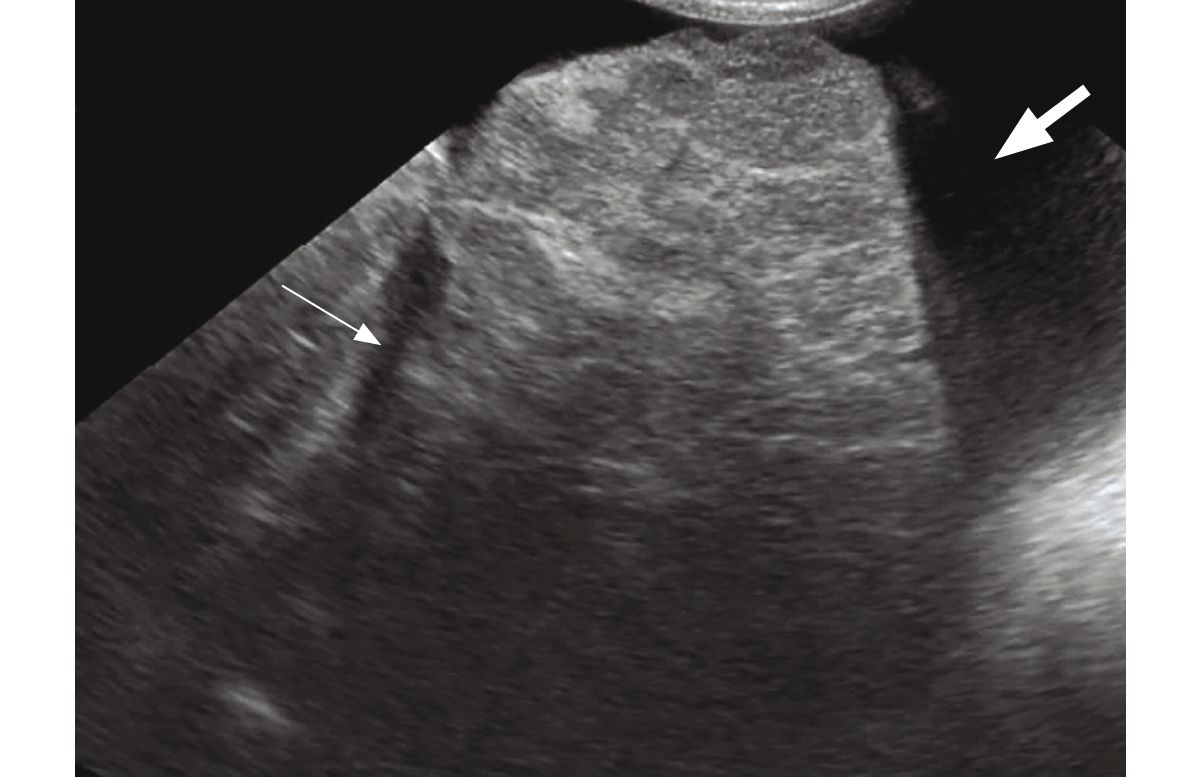
Radiology of the liver in affected dogs is usually normal, so ultrasound is a required part of the routine work-up in all dogs with suspected CH. A summary of the ultrasound changes described in literature is shown in (Table 3). It is important to note that several studies have shown that there are no ultrasonographic criteria that can predict the presence of CH; in fact the liver may appear normal on a scan, even in the presence of significant disease [17] [18] [19].
| Abnormality | % dogs showing change |
|---|---|
| Microhepatica | 39 |
| Ascites | 29 |
| Heterogenous/non-homogenous/mottled | 23 |
| Hyperechoic | 18 |
| Nodular | 17 |
| Irregular margins | 17 |
| Normal | 14 |
| Hepatomegaly | 7.8 |
| MAPSS (multiple acquired portosystemic shunts) | 4.3 |
| Enlarged hepatic lymph nodes | 2.8 |
| Hypoechoic | 2 |
Table 3. Ultrasound changes seen in chronic hepatitis.
In advanced CH, the liver may be small and irregularly marginated (Figure 3) on ultrasound, with signs of portal hypertension. These include ascites, edema (especially visible in the gallbladder and pancreas), reduced portal flow velocity (mean velocity of < 10 cm/s, as compared to a normal range of 10.5-25.7 cm/s) or hepatofugal flow and the visualization of MAPSS, usually seen as a complex plexus of small tortuous vessels caudal to the left kidney [20].
Biopsy acquisition
The diagnosis of CH requires hepatic tissue sampling. Fine-needle aspirates are not adequate to make a diagnosis, and often result in misclassification of the disease process. Percutaneous ultrasound-guided biopsy with large (14 or 16G) needles can provide adequate samples for diagnosis if multiple cores are taken [21]. The diagnostic accuracy of 18G needle biopsies, however, is questionable, as these result in a relatively small sample size, are subject to fragmentation when fibrosis is present, and may not enable sampling of abnormalities located in lobes other than the readily accessible left medial or lateral lobes. This is a problem, since there is often heterogeneity between different liver lobes in terms of histological severity and copper deposition. In general, accurate diagnosis of CH requires the pathologist to evaluate 10-12 portal regions, which is hard to attain unless multiple percutaneous biopsies are procured. Multiple biopsies can, however, increase the risk of bleeding.
Laparoscopy is the preferred technique to obtain hepatic biopsies. This enables gross evaluation of the entire liver, the extrahepatic biliary system and surrounding structures while permitting acquisition of multiple large specimens that consistently result in samples with a mean of 16-18 portal triads per biopsy specimen. For the diagnosis of CH, five samples from at least two different lobes are obtained; three for histopathologic evaluation and one each for aerobic/anaerobic culture and heavy metal quantification.
| Assessment parameter | High-risk criteria |
|---|---|
| PCV | < 30% |
| Platelet count | < 80,000 |
| PT/aPTT | > 1.5 x upper limit normal |
| vWF (in susceptible breeds) | < 50% |
| Buccal mucosal bleeding time (BMBT) | > 5 minutes |
| Fibrinogen | < 100 mg/dL |
Box 2. Assessment of bleeding risk for hepatic biopsy.
Risks of biopsy include anesthetic complications (especially in patients with advanced liver disease), hemorrhage, air embolism (on laparoscopy), infection, pneumothorax and vagotonic shock. The primary concern is hemorrhage [22]. Assessment of the bleeding risk in dogs with liver disease that have deficiencies in both pro- and anti-coagulants as well as in regulators of fibrinolysis is difficult. Prolongations of PT and aPTT occur in about 40% of affected dogs. Decreased fibrinogen, anti-thrombin and protein C activity also occur in many dogs, and mild anemia and thrombocytopenia are occasionally present. Based on the human literature and the limited work to date in dogs, guidelines for bleeding risk assessment are outlined in (Box 2) [13] [21] [22].
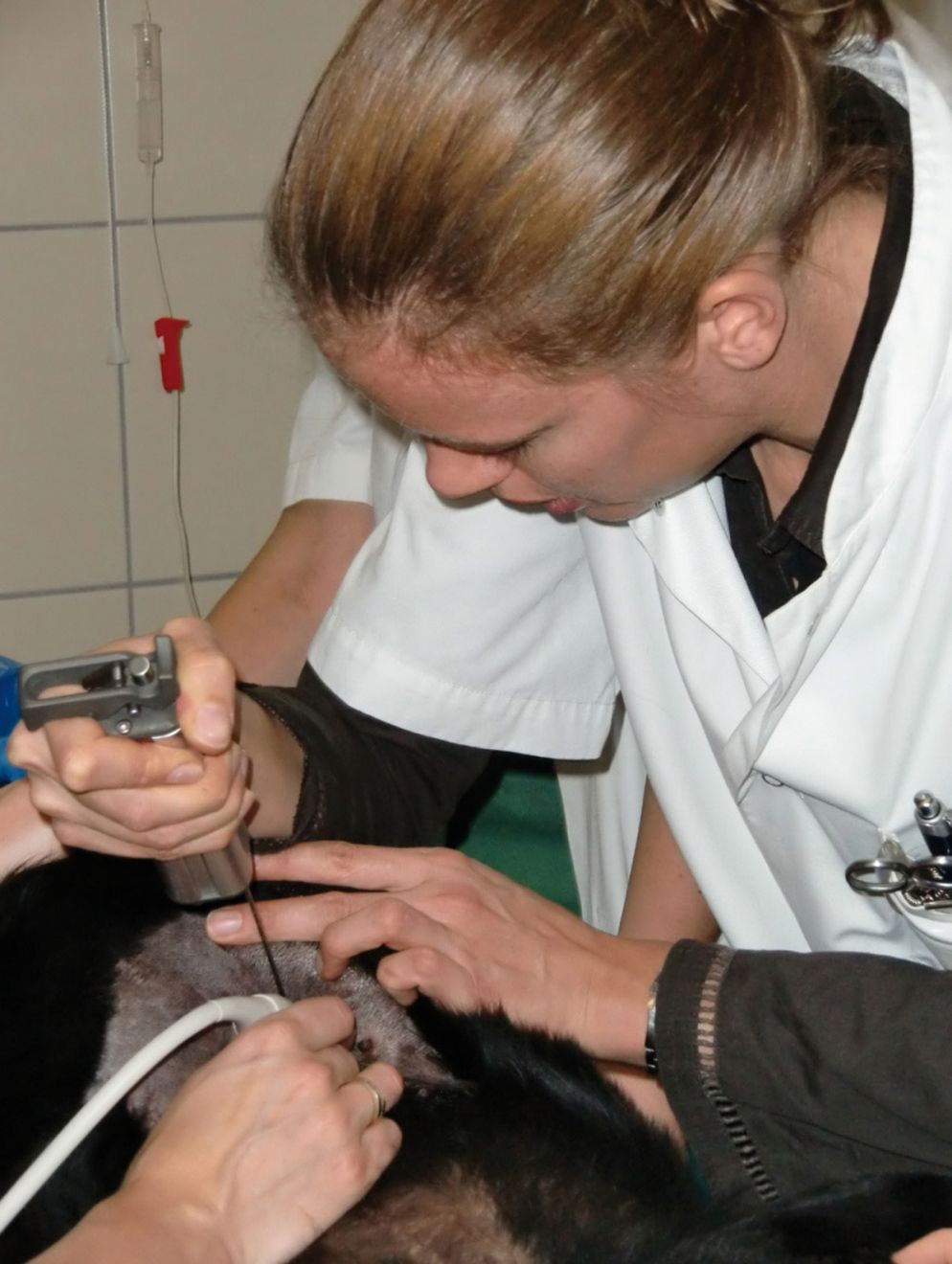
Percutaneous ultrasound-guided liver biopsy (Figure 4) carries a greater bleeding risk than techniques in which hemostasis can be controlled locally (e.g., laparoscopy), although the risk of complications (defined as the need for transfusion or fluid resuscitation) appears to be low for both methods, at around 1-5% [13] [22].
When high-risk patients are identified, it is unknown if therapeutic interventions – such as administration of blood products or vitamin K – will decrease the risk of hemorrhage following hepatic sampling. The exception is the dog with low von Willebrand factor activity, which should be given cryoprecipitate and desmopressin. Thus the recommendation in high-risk dogs is to pay strict attention to technique and monitor them carefully in hospital after the biopsy procedure for 12 hours, and to be prepared to rescue with blood product support if necessary [13].
Biopsy interpretation
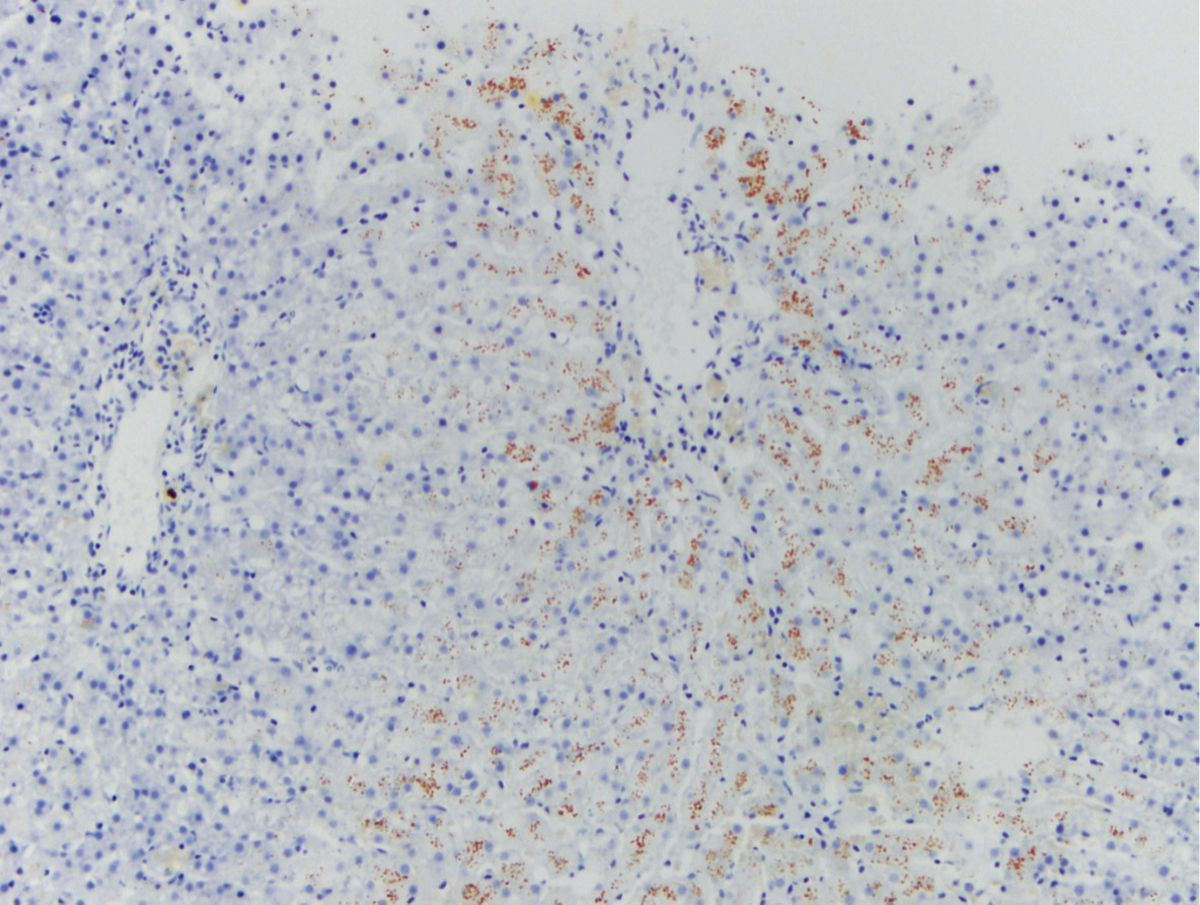
Evaluation of liver biopsy specimens requires H&E, Sirius red or Massons’ trichrome (for fibrosis) and Rhodanine (for copper) staining [21]. The pathologist should comment on the type, location and quantity of inflammation, fibrosis, degenerative change (lipidosis, vacuolar change, lipogranulomas), the presence, location and extent of cell death and ductular reaction, and the lobular distribution and amount of copper staining (Figure 5). Special stains for infectious organisms are indicated in some cases, particularly in pyogranulomatous hepatitis. An exchange of information between the clinician and the pathologist may be necessary to maximize the value obtained from biopsies. In some cases, evaluation of samples by a pathologist (and internist) with expertise in hepatic histopathology and medicine should be considered.
Treatment
Treatment is targeted to etiology. Suspected infectious agents should be treated with appropriate antimicrobial therapy, and toxic agents or drugs eliminated from the dog’s environment. Any increase in hepatic copper in a dog with CH should be treated. Treatment of Cu-CH is summarized in Table 4 and involves dietary Cu restriction and methods to chelate Cu or prevent intestinal absorption (penicillamine and zinc) [7]. Concurrent administration of hepatoprotectant and anti-oxidant medications is indicated (S-adenosylmethionine, vitamin E +/- ursodeoxycholate). Some dogs with Cu-CH have intense inflammatory infiltrates and may benefit from a short course of anti-inflammatory corticosteroids.
| Drug and dosage | Mechanism of action and notes |
|---|---|
| Cu-restricted diet Feed appropriate commercial or home-made diet with < 5 mg/kg dry weight (0.1-0.12 mg/100 kcal) < 0.1 µg/g Cu in water; use distilled water or test water for Cu |
Limits intestinal absorption of Cu Available Cu restricted diets often have unnecessary protein restriction; consider additional protein Most dogs need lifetime low Cu diet With Cu pipes can run water for a few minutes to eliminate Cu |
| D-Penicillamine 10-15 mg/kg q12H PO on an empty stomach |
Cu chelator Common side effects include nausea and vomiting. Rarer side effects include Cu, Fe or Zn deficiency, vitamin B12 deficiency, skin eruptions, proteinuria and hematologic dyscrasias. May cause a mild increase in serum ALP and vacuolar hepatopathy. Do not give with zinc |
| Zinc (zinc gluconate) 50 mg q12H on an empty stomach |
Induces synthesis of cytoplasmic metallothionein in intestine and liver, decreasing Cu absorption and protecting the liver. Removes copper slowly, so only appropriate for maintenance Commonly causes nausea and vomiting; hemolytic anemia is seen rarely. Serum levels must be monitored; should be > 200 mg/dL but less than 1000 mg/dL |
| S-Adenosylmethionine (SAMe) 20 mg/kg PO q24H on an empty stomach |
Increases glutathione levels (GSH), promotes anti-inflammatory polyamines and methylation of DNA and membranes to promote cell stability Occasionally causes vomiting; since the compound is unstable, use products with proven pharmacodynamics in the dog |
| Vitamin E 10 IU/kg PO q24H, not to exceed 400 IU/dog/day |
Anti-oxidant: prevents lipid peroxidation of membranes Give with food. Can be pro-oxidant and interfere with coagulation at high doses |
| Ursodiol 10-15 mg/kg PO q24H given with food. |
Choleretic, anti-oxidant and anti-apoptotic. Indicated with hyperbilirubinemia or evidence of ultrasound changes in biliary tree. Occasionally causes vomiting. Generic formulations generally have good bioavailability |
Table 4. Treatment of copper-associated chronic hepatitis.

Bedlington Terriers, Dalmatians and young dogs with markedly elevated (> 3000 µg/g DW) hepatic Cu will likely need lifetime dietary therapy combined with Cu chelation. In other dogs, the time necessary to achieve normal Cu balance with penicillamine and low copper diet is poorly defined. Some work in Labrador Retrievers suggests that duration of chelation is related to initial hepatic Cu concentration, with about 6, 9 and > 12 months required for 1000, 1500 and 2000 µg/g DW respectively (Figure 6). Whether this is true for other breeds is unknown. Expert opinion is that some dogs “de-copper” more easily than others and that this is often independent of hepatic Cu concentration [13].
Ideally, re-biopsy with qualitative and quantitative Cu determination should define when chelation can be stopped. If this is not possible then serum ALT is used as a surrogate marker, whilst recognizing that levels can be normal despite ongoing histologic inflammation; chelation should therefore be continued 2-3 months beyond normalization of serum ALT. Although some limited work suggests that fine-needle aspirates stained with rhodamine may be useful in monitoring hepatic copper, this is not recommended until further studies are done.
In some dogs, normal Cu balance is restored yet serum ALT and histologic evidence of inflammatory disease persists. These dogs either did not have Cu-CH, or the damage has exposed neoepitopes and incited a self-perpetuating immune disease.
Typically, all affected dogs stay on a Cu-restricted diet, but this is often insufficient to maintain normal hepatic Cu, although it is hard to predict which dogs will need additional therapy. In general dogs with high initial hepatic Cu (> 2000 µg/g), dogs with a family history of Cu-CH, and dogs in which serum ALT does not normalize within a 6-8 month period of chelation will often receive an appropriate diet in combination with maintenance penicillamine or zinc therapy.
As histologic improvement lags behind clinical and laboratory improvement, treatment recommendation adjustments must go beyond laboratory remission by several months before attempting treatment withdrawal.
Limited studies suggest that some dogs with idiopathic Cu-CH truly have immune disease and will go into remission on appropriate therapy, although prospective clinical trials of immunosuppression in suspected immune CH are lacking. Corticosteroids, azathioprine, mycophenolate and cyclosporine have been advocated to treat presumptive immune hepatitis in dogs (Table 5), although again none have been evaluated in prospective clinical trials. Often dogs with presumptive immune disease are also placed on concurrent hepatoprotectant medications.
| Drug and dosage | Comments and possible side effects |
|---|---|
| Azathioprine 1 mg/kg PO q24H for 7 days then 1 mg/kg q48H |
Increase in serum liver enzymes (typically reversible upon discontinuation) Reversible bone marrow suppression |
| Prednisolone 2 mg/kg PO q24H (no greater than 40 mg/day) tapered to 0.5 mg/kg q48H |
PU/PD/polyphagia GI upset Hypercoagulability Induction of serum ALP and GGT Development of steroid hepatopathy Increased susceptibility to infections (e.g., UTI) Catabolism Sodium retention Use dexamethasone in patients with ascites |
| Cyclosporine 5 mg/kg PO q12H |
Nausea/vomiting Gingival hyperplasia Increased susceptibility to infections (e.g., UTI and opportunistic fungi) Use emulsified preparations only Initial therapy should not be with generic products |
| Mycophenolate 10 mg/kg PO q12H |
Diarrhea |
Table 5. Immunosuppressive therapy for presumptive immune-mediated chronic hepatitis.
Again, the optimum therapeutic endpoint for judging treatment response is normalization of hepatic histology, but this is often impossible, so ALT activity may be used as a surrogate marker. The timeframe for remission in dogs with immune CH is unknown. Control of enzymes may take 2-3 years in humans, although better long-term response is seen when enzyme activity is controlled within 3 months. As histologic improvement lags behind clinical and laboratory improvement (in humans by 3-8 months), treatment recommendation adjustments must go beyond laboratory remission by several months before attempting treatment withdrawal. Stable laboratory data within reference intervals over 12-18 months may be adequate to consider tapering medication. The relapse rate in dogs is unknown but in humans is up to 50%. Recapture of disease control with re-induction of primary therapy is often promptly achieved.
Prognosis and complications
Once diagnosed, dogs with CH typically experience progressive disease. Survival times have been reported in several studies, all of which studies were retrospective [13], and dogs were treated with a variety of medications and diets. In 10 studies with survival data (n=364 dogs) the mean survival was 561 days +/- 268 days. In dogs with biopsy-proven cirrhosis, survival was considerably less, 23 +/- 23 days (n=39). The clinicopathologic factors associated with a poor prognosis are hyperbilirubinemia, increased PT and aPTT, and hypoalbuminemia. The presence of ascites and the degree of fibrosis on biopsy are also negative prognostic signs; the one exception may be in Cocker Spaniels with CH, where dogs with ascites can have prolonged survival.
Complications of CH in the dog include portal hypertension, ascites, hepatic encephalopathy, gastrointestinal ulceration and coagulopathies (both bleeding and thrombosis) [20] [23] [24]. Bleeding is more common with end-stage disease, and thrombosis occurs more often where other pro-thrombotic factors are involved, such as systemic inflammation, surgery or corticosteroid therapy [20]. The incidence of secondary bacterial infection is poorly documented in dogs with CH, but appears to be low at around 5% [24].
Chronic hepatitis can occur in any breed of dog and the onset can be insidious; significant histological lesions can exist in the absence of accompanying serum liver enzyme elevation. Multiple biopsy samples are required for a definitive diagnosis, although biopsy can carry some risk for the patient. Targeted treatment is preferred wherever possible, although the causative agent may not be identified in many cases, and therapy should be continued for some months after clinical signs resolve.
References
- Van den Ingh TSGAM, Van Winkle TJ, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver: Hepatocellular death, hepatitis, and cirrhosis-2 (updated version) In: WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Society of Comparative Hepatology. Available at; http://www.vetvisuals.com/lms/moodle/mod/book/view.php?id=1001&chapterid=52859
- Johnston AN, Center SA, McDonough SP, et al. Hepatic copper concentrations in Labrador Retrievers with and without chronic hepatitis: 72 cases (1980-2010). J Am Vet Med Assoc 2013;242:372-380.
- Strickland JM, Buchweitz JP, Smedley RC, et al. Hepatic copper concentrations in 546 dogs (1982-2015). J Vet Intern Med 2018;32(6):1943-1950.
- Subcommittee on Dog and Cat Nutrition, Committee on Animal Nutrition, National Research Council. Nutrient Requirements of Dogs and Cats. Washington, DC: The National Academy Press; 2006.
- Webster CRL, Center SA, Cullen JM, et al. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J Vet Intern Med 2019;33(3):1173-1200.
- Fieten H, Hooijer-Nouwens BD, Biourge VC, et al. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in Labrador Retrievers. J Vet Intern Med 2012;26(6):1274-1278.
- http://www.fediaf.org/images/FEDIAF_Nutritional_Guidelines_2019_Update_030519.pdf
- Watson P. Canine breed-specific hepatopathies. Vet Clin North Am Small Anim Pract 2017;47:665-682.
- Kemp SD, Panciera DL, Larson MM, et al. A comparison of hepatic sonographic features and histopathologic diagnosis in canine liver disease: 138 cases. J Vet Intern Med 2013;27:806-813.
- Feeney DA, Anderson KL, Ziegler LE, et al. Statistical relevance of ultrasonographic criteria in the assessment of diffuse liver disease in dogs and cats. Am J Vet Res 2008;69:212-221.
- Warren-Smith CM, Andrew S, Mantis P, et al. Lack of associations between ultrasonographic appearance of parenchymal lesions of the canine liver and histological diagnosis. J Small Anim Pract 2012;53:168-173.
- Twedt DC. Reactive hepatopathies and chronic hepatitis in the dog. Vet Q 1998;2:S46-47.
- Buob S, Johnston AN, Webster CR. Portal hypertension: pathophysiology, diagnosis, and treatment. J Vet Intern Med 2011;25:169-186.
- Lidbury JA. Getting the most out of liver biopsy. Vet Clin North Am Small Anim Pract 2017;47:569-583.
- Webster CR. Hemostatic disorders associated with hepatobiliary disease. Vet Clin North Am Small Anim Pract 2017;47:601-615.
- Rothuizen J. Important clinical syndromes associated with liver disease. Vet Clin North Am Small Anim Pract 2009;39:419-437.
- Wagner KA, Hartmann FA, Trepanier LA. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998-2003. J Vet Intern Med 2007;21:417-424.
- Bexfield N. Canine idiopathic chronic hepatitis. Vet Clin North Am Small Anim Pract 2017;47:645-663.
- Poldervaart JH, Favier RP, Penning LC, et al. Primary hepatitis in dogs: a retrospective review (2002-2006). J Vet Intern Med 2009;23:72-80.
- Kearns S. Infectious hepatopathies in dogs and cats. Top Companion Anim Med 2009;24:189-198.
- Bunch SE. Hepatotoxicity associated with pharmacologic agents in dogs and cats. Vet Clin North Am Small Anim Pract 1993;23(3):659-670.
- Dirksen K, Fieten H. Canine copper-associated hepatitis. Vet Clin North Am Small Anim Pract 2017;47:631-644.
- Thornburg LP, Rottinghaus G, McGowan M, et al. Hepatic copper concentrations in purebred and mixed-breed dogs. Vet Pathol 1990;27:81-88.
- Thornburg LP, Rottinghaus G, Dennis G, et al. The relationship between hepatic copper content and morphologic changes in the liver of West Highland White Terriers. Vet Pathol 1996;33:656-661.
Cynthia RL Webster
DVM, Dipl. ACVIM (SAIM)
United States
Dr. Webster graduated from Cornell University in 1985 and after working in private practice returned for residency training at Cummings School of Veterinary Medicine. She was board-certified in small animal internal medicine in 1993 and then did a post-doctoral fellowship in hepatocyte transport biology. Currently Professor and Associate Chair in the Department of Clinical Sciences at Tufts Veterinary School, she has authored over 100 peer reviewed manuscripts as well as several book chapters. Most recently, she chaired the ACVIM Consensus Panel on the diagnosis and treatment of chronic hepatitis in the dog.
Other articles in this issue
Share on social media