How I approach... The cat with chronic diarrhea
Written by Craig B. Webb
Clinicians will be all-too-familiar with the cat that has recurrent diarrhea; dealing with these patients can be frustrating for both the veterinarian and the owner, but Craig Webb offers his thoughts on how best to approach these cats with a case-based article that illustrates the key pointers for a successful outcome.

Key Points
The clinician can employ a variety of approaches when dealing with a cat that has chronic diarrhea. Two of the most useful methods are Clinical Reasoning and Script Recognition.
First approach the case as a clinician; diagnostic testing should arise from a clinical diagnosis.
Important incongruities and key features are to be found in the signalment, history, and physical exam.
Defining the problem in an accurate, complete and concise manner aids in diagnosis.
Positive predictive value is a function of the prevalence of the disease in the population being tested.
Diet is a critical component in both the diagnosis and the treatment of cats with chronic diarrhea.
Introduction
There is a significant difference between the way a clinical sign or a disease process is organized within a textbook, and the way a cat with that particular clinical sign or disease process actually presents to you as a clinician. Therefore, although an understanding of the “textbook” case is critical, it is still a very long way from an understanding of the cat on the examination table in front of you. What follows is my attempt to describe what is actually happening between that cat and me as the clinician, as I try to achieve that understanding.
The approach
My approach to a cat with chronic diarrhea – which is defined as continuous or intermittent diarrhea (reduced consistency, increased volume, or increased frequency) of greater than 3 weeks’ duration – may actually come from a variety of different directions. Consider the following options:
• I like to start with the cat and the owner together. I use the history of the cat, the history of the clinical sign(s), and a physical examination to rank-order, from most likely to least likely, my list of differentials for feline chronic diarrhea. From that list, I prioritize the diagnostic test(s) that seems best suited to confirm or refute my number one differential. Additional diagnostic testing will move a possible diagnosis either up or down my rank-ordered list until I identify the differential that sticks to the top. This is known as the Clinical Reasoning Approach, where one moves logically from a presumptive to a definitive diagnosis.
• The next approach is much less involved. Again I start by looking at the cat and listening to the owner. Then I look at the case presentation, or “illness script”, and simply go with what my instinct tells me. This is known as the Script Recognition Approach and is based very strongly on “gut feeling”.
• As I review the medical history and perform the physical examination, I pay special attention to aspects of the case that do not make sense or do not seem to fit; these incongruities often turn out to be important clues. I will also run a “Cine Loop” of the case through my head, from start to finish, attempting to describe the case more completely and accurately each time, in search of any missing piece of the puzzle. These are components of the Key Features Approach, which separates critical notes from background noise.
• Finally, despite the eloquence of the argument for a committed work-up, and often as a result of financial constraints, the owner may opt to begin a “trial treatment”. So I prescribe treatment X and schedule a recheck in 2 weeks. This is known as the Ready-Fire-Aim Approach, and frequently evolves into the Ready-Fire- Fire-Fire Approach.
Many variables can influence the way I approach a case; some in a positive manner, some in a way that results in a (somewhat predictable) error of medical judgement. The above methods are not mutually exclusive – in many cases one approach can complement another. I strongly encourage you to “think about how you think about cases” [1] and this is best illustrated by considering some case presentations.
Case presentation #1
I start with the information as shown in the appointments schedule; it will usually simply give details of a cat with a certain signalment with the presenting complaint of “chronic diarrhea”. Beginning with just the signalment and presenting complaint, I begin to form an “illness script” or picture in my head of the case. If the schedule tells me I am about to see a kitten with chronic diarrhea, my illness script is very different than if I know I am about to see a 14-year-old Siamese with chronic diarrhea (Table 1). When I actually see the cat, perform a physical examination, and take a history from the owner I use that information to fill in details and enhance the clarity of my picture. At this point in my approach, I form a presumptive diagnosis using Script Recognition.
| Signalment, presenting complaint, history, physical examination | ||||
| Signalment: age, gender, breed | ||||
| Age | ||||
| Kitten | Adult | Geriatric | ||
| Primary GI > secondary GI | Primary GI & secondary GI | Primary GI < secondary GI | ||
|
• Dietary
• Stress |
• Food responsive • I BD • G I LSA • Infectious • Ileus |
• CKD • Pancreatitis • Neoplasia • Cholangitis • Hyperthyroidism • EPI |
Intestinal neoplasia | Extraintestinal neoplasia |
| All etiologies listed in Adult section | ||||
Table 1.Building an “Illness Script” for cats with chronic diarrhea: an animal’s age has a major impact on the etiology
GI = gastrointestinal; IBD = inflammatory bowel disease; GI LSA = gastrointestinal lymphosarcoma; EPI = exocrine pancreatic insufficiency; CKD = chronic kidney diseasex
As simple as it sounds, it has been shown that the more experienced a clinician is, the larger the role Script Recognition plays in their approach to cases. The power of this approach depends on how accurate and complete the illness script is, and my ability, through experience, training, and recall to recognize and identify that specific script.
A “cat with chronic diarrhea” could have almost anything. However, a 5-month-old F/S domestic shorthair (Signalment) with chronic intermittent large bowel diarrhea (Presenting complaint and History), adopted from the shelter and otherwise healthy (History), with a BCS of 5/9 and mild perianal inflammation (Physical exam), unresponsive to repeated courses of metronidazole and fenbendazole (History) is a case of Tritrichomonas foetus* until proven otherwise [2] (Figure 1).

*Tritrichomonas foetus may soon be renamed T. blagburni, based on molecular testing, host specificity, and pathology. This is simply to distinguish the feline T. foetus organism from the bovine organism and has no impact on the diagnosis or treatment of feline trichomonosis.
In this case, the Ready-Fire-Aim approach had already resulted in several trial treatments from the referring clinician, with a broad-spectrum antihelminthic (fenbendazole 50 mg/kg Q24 h for 5 days) and metronidazole benzoate (25 mg/kg Q24 h for 7 days). This would be standard practice in kittens, given the prevalence of parasites in a shelter population of this age group, and here the lack of response to these interventions is a Key Feature of the illness script.
Another important feature of my illness script in this case is to establish whether the diarrhea was predominantly small bowel or large bowel in origin (Table 2). Frequently, the answer is “mixed”, and there is a significant overlap in etiology for both categories. But the distinction turns out to be important in this case, given the fact that this kitten was not afflicted by GI parasites susceptible to standard antihelminthics. That leaves T. foetus and a drug-resistant Giardia spp. as my top infectious ruleouts, and with signs that indicate large bowel diarrhea I favor the former option.
| Clinical sign | Small bowel | Large bowel |
| Mucus | Absent | Common |
| Fresh Blood | Absent | Common |
| Melena | +/- | Absent |
| Volume | Increased | Normal, decreased |
| Character | Soft to watery | Soft to formed |
| Frequency | Normal, slight increase | Increased |
| Dyschezia | Absent | +/- |
| Tenesmus | Absent | +/- |
| Urgency | Absent | Common |
| Weight loss | Common | Uncommon |
| Vomiting | +/- | Uncommon |
| Appetite | Variable | Often normal |
| Activity | Often decreased | Often normal |
| Borborygmus | +/- | Absent |
| Flatulence | +/- | +/- |
Fecal examination (Figure 2) would be an obvious and important diagnostic step when working up most cases of feline chronic diarrhea, especially with this age group and environmental history. The diagnostic techniques available for examination of feces are beyond the scope of this article, but a number of excellent resources are available to help clinicians make sound diagnostic choices** [3].

**Companion Animal Parasite Council (CAPC)™ www.capcvet.org
The importance of dietary intervention in cases of chronic diarrhea will be emphasized a number of times in this paper, and I will also emphasize diet as a diagnostic tool. Considering the likelihood of diet-related diarrhea in kittens (Table 1), a diet trial would certainly have been a strong consideration in this case. The use of hypoallergenic and hydrolyzed diets will be discussed as we move to an older age group, but in this kitten I would have reached for a highly digestible diet [4], or (because the problem was large bowel diarrhea) possibly a GIfiber diet [5], whilst bearing in mind the caloric requirements of a growing kitten. My preferred fiber source as a non-specific treatment for diarrhea is psyllium (unflavored powder, 425 mg per 1/8 tsp; 0.25-0.5 tsp per meal), a soluble fiber with evidence-based support for use in canine large bowel diarrhea cases [6].
Expanding the definition of dietary intervention beyond a particular food, I would strongly consider supplementing this particular kitten with a probiotic. Whether a cause or consequence, an unbalanced intestinal microbiome, known as dysbiosis, is likely a very important contributor to gastrointestinal disease and the associated clinical signs in both people and pets. One study showed that giving shelter cats a probiotic significantly reduced the number of days the cats experienced diarrhea [7]. Whilst ronidazole is the treatment of choice for T. foetus diarrhea (30 mg/kg Q24 h for 14 days) [8], it appears that combining ronidazole with a probiotic might reduce the likelihood that cats will relapse, as is otherwise frequently the case [9]. Although our ability to assess or monitor the microbiome is currently quite limited, at least one laboratory has recently developed and commercialized a fecal “Dysbiosis Index” test***. Employing this test may help me further refine the illness script, as well as help monitor therapies in cases of chronic diarrhea. As a cautionary note, at least one study has shown that there can be a huge discrepancy between what is on the label and what is inside the bottle when it comes to OTC probiotics [10], and I stick with trusted brands produced by companies with deep roots in veterinary medicine.
*** Gastrointestinal Laboratory, Texas A&M University – although the dysbiosis test is currently only validated for dogs, recent evidence suggests that it may be useful for cats as well.
In this case, I incorporated a number of different approaches, avoided a variety of potential medical errors in judgement, and with a T. foetus-positive fecal PCR result, treated the kitten with ronidazole, a highly digestible diet, psyllium and a probiotic – putting an end to the chronic diarrhea.
Case presentation #2
My next appointment is a 3-year-old F/S domestic shorthair (Signalment) with chronic intermittent small bowel diarrhea (Presenting complaint and History), adopted from the shelter and otherwise healthy except for the occasional hairball vomit (History), a BCS score of 4/9 with mild interdigital inflammation (Physical exam), unresponsive to repeated courses of metronidazole and fenbendazole (History) (Figure 3).

Laboratory tests are PCR-positive for T. foetus. I am pleased to get a positive result with minimal financial outlay, and since I have just experienced success treating Case #1 with ronidazole I naturally prescribe the same drug for this cat with chronic diarrhea – but to no effect.
This example highlights how my approach to a case can be significantly impacted by my success or failure with previous cases, previous diagnostics, and previous treatments. That makes sense; we are supposed to learn from our experiences. Unfortunately, in this example, the influence of my recent success affected my focus in constructing an illness script. Case #2 involves a young adult cat, not a kitten; this cat was experiencing small bowel diarrhea, not large bowel; not all cats from shelters have parasites; the vomiting of hairballs was seen as incidental; the BCS was 4/9; the mild interdigital inflammation was regarded as incidental; and the failure to respond to dewormers was interpreted as supporting T. foetus... after all, the laboratory diagnostic test result said so.
This case also highlights what I believe to be a critical component in my work-up of cases in veterinary medicine: the appropriate placement of diagnostic testing. Testing for infectious agents associated with feline chronic diarrhea is a prime example; study after study reminds us that using a diagnostic test to identify an organism does not equate to identifying the cause of the diarrhea. Even in the face of advanced technologies such as PCR-based identification of intestinal parasites, clinical reasoning is crucial for successful treatment [11]. So when do I use diagnostic testing, and what tests do I use in my approach to a cat with chronic diarrhea?
Positive predictive value is a function of the prevalence of the disease in the population which I am testing. Each individual cat becomes part of a “population” of patients that I decide to test for this or that... or not test for this or that. The better I am at correctly identifying those patients that likely have disease X, the higher the prevalence of disease X in my “population” of patients. Therefore, the value of the diagnostic test I request and my ability to confidently interpret the result of the test depends on my ability as a clinician to make a clinical diagnosis before ordering a diagnostic test. In summary: my diagnostic test results are only as good as I am!
So back to Case #2, where ronidazole had no effect. Discouraged by my treatment failure, I turn to the literature on feline chronic diarrhea in the hopes of finding a more successful approach to this case. A recent series of articles describe the diagnosis of chronic small bowel disease in adult cats and the intestinal histology in cats suspected of having chronic small bowel disease [12] [13]. A key component of the diagnostic work-up in these cases was abdominal ultrasound, which frequently demonstrated thickening of the small intestines. Subsequent full-thickness biopsies revealed that about half of the cats had a diagnosis of chronic enteritis and most of the other cats had a diagnosis of GI lymphoma. So one scenario for this cat is that I perform abdominal ultrasound, find thickened small intestines, obtain endoscopic small intestinal biopsy samples for histopathology, diagnose lymphocytic plasmacytic enteritis (IBD), and start the cat on prednisolone.
But before I settle on that approach, I start my Cine Loop review of the illness script. I “walk through” the case again and again, looking for incongruities and key features that I may have missed. I ask myself “What if the same cat’s presenting complaint had been interdigital inflammation?”. A young adult cat with itchy inflamed digits is an illness script consistent with allergy. Now I add in the GI signs, and using Clinical Reasoning the rule-out that rises to the top of my list is food allergy. The diagnostic test of choice for food allergy is not abdominal ultrasound or intestinal biopsies, but a food trial.
A critical series of papers on cats with chronic diarrhea [14] [15] described a significant number of cats (30%) presenting for chronic GI signs (diarrhea or vomiting), pruritus, or both; their clinical signs were resolved with an elimination diet trial, using a commercial canned singlesource protein hypoallergenic diet. The authors use the term “food sensitivity” to characterize the etiology behind chronic diarrhea in these cats, including food intolerance and food allergy. Of clinical importance, the food sensitivity cats in these studies showed resolution of GI signs after just a 2-week trial of the hypoallergenic diet. The diagnostic work-up of these cats was extensive. In fact, 50% of the cats who were diagnosed with food sensitivity had histopathology that described mild to severe lymphoplasmacytic enteritis, i.e., inflammatory bowel disease. Ironically, although abdominal radiographs were performed to rule out GI obstruction and abdominal masses, abdominal ultrasound was not part of the diagnostic work-up of these cats.
The message for me is that when I approach an otherwise healthy (i.e., no evidence of secondary GI disease) and stable (i.e., no significant weight loss or decrease in appetite) young adult or adult cat presenting for chronic diarrhea, I think “food first” as an appropriate early diagnostic tool. I may prepare the owner for several sequential 2-week diet trials should the original diet fail. I start with a prescription novel protein or hydrolyzed diet (food allergy), as there does not appear to be a significant clinical difference between the two [16]. If that fails, I consider an easily digestible diet (evidence-based) or a GI fiber diet (if large bowel) [17] [18]. Finally, I might employ a bespoke elimination diet in the hopes of identifying a single offending ingredient.
Case presentation #3
| Age | Etiology* |
| Young | Infectious |
| Young adult | Food |
| Adult | Inflammation |
| Older Adult | Neoplasia |
*The dashed lines between entries emphasize the overlap between etiologies.
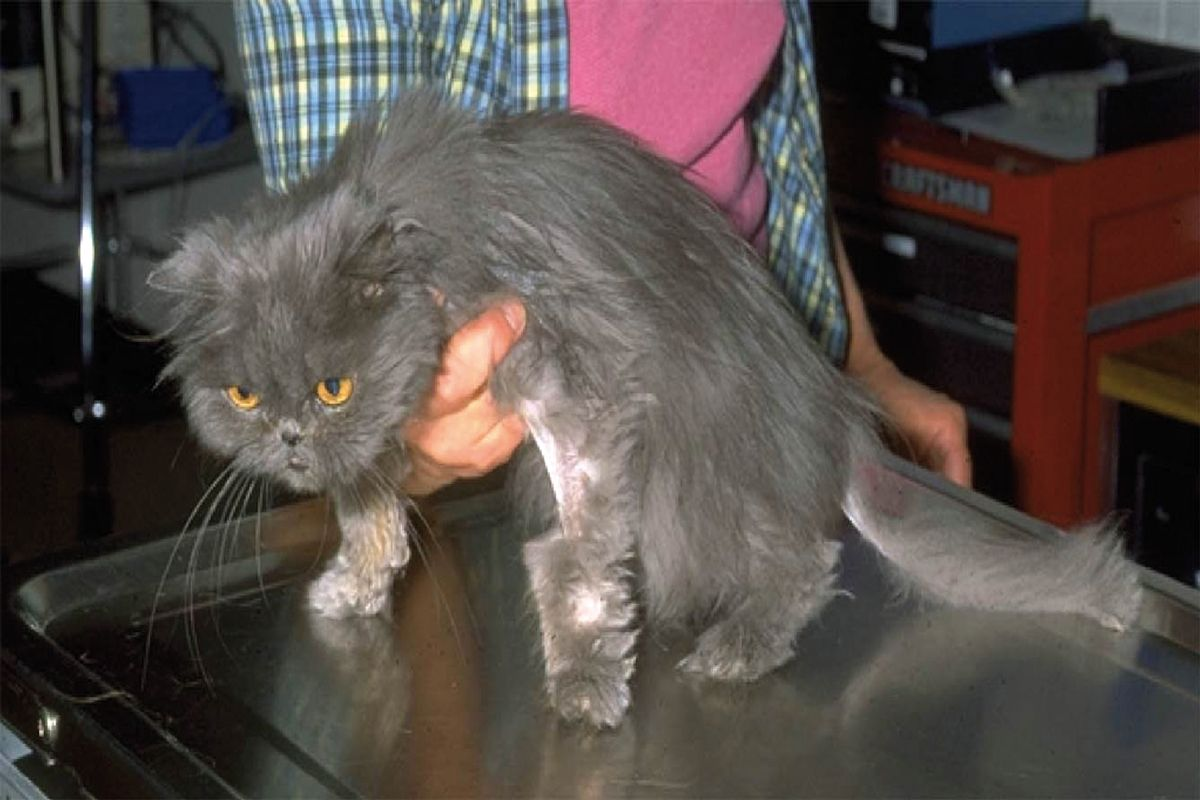
When I consider adult and older cats with chronic diarrhea (Table 3), or if I am faced with young or young adult cats where the chronic diarrhea appears to be a local sign of a more systemic and serious problem, my approach becomes more aggressive, both in terms of the time-frame and the diagnostics. Although food sensitivity and infectious causes of chronic diarrhea can cause systemic signs, they are much lower on my list for a cat that is more seriously ill. Case #3 is a 12-year-old M/N Persian with chronic small bowel diarrhea, including significant weight loss and poor body condition (Figure 4). Here, the Ready-Fire-Fire-Fire approach with prophylactic deworming, diet trials, supplements or best-guess antibiotics is no longer appropriate. In this situation, since secondary GI causes of diarrhea become more prominent with age (e.g., linked to liver, pancreas or thyroid problems, etc.), I will attempt to rule out those that warrant diagnostic investigation. Then, if I have done my job as a clinician, the case probably comes down to my attempting to distinguish between IBD and GI lymphoma. I start with an illness script and Script Recognition – does this cat look and feel like it has cancer (cachectic, muscle wasted, thickened intestines) and does it act as if it has cancer (lethargic and hyporexic)?
Then I apply Clinical Reasoning, paying attention to incongruities and key features – does it make sense that the clinical signs of GI lymphoma were first recognized two years ago? Does it make sense that IBD has led to a 35% loss of body weight over 2 months? Does it make sense that the cat is cachectic in the face of polyphagia? Could the cat have more than one significant problem, as in cases of feline triaditis?
I will check a TT4 for thyroid function and run a fasted panel for folate, cobalamin, fTLI, and fPLI. Low levels of folate and cobalamin are consistent with proximal and distal small intestinal disease, respectively. A disparity between the two, high folate and low cobalamin, is consistent with some degree of dysbiosis. An elevation in fPLI is consistent with pancreatitis, although I would look closely for additional clinical signs such as dysrexia and lethargy, or elevations in blood glucose and total bilirubin. Finally, although exocrine pancreatic insufficiency is rare in cats, it can cause chronic small bowel diarrhea with weight loss, usually despite a good appetite [19]. For me, cobalamin is usually the most informative value on the GI panel [20]; low values are associated with significant small intestinal disease, and really low values may be associated with GI lymphoma [21]. In addition, cobalamin can be easily supplemented (see Table 4).
| Drug | Mechanism | Indication | Dose | Side effects |
| Prednisolone | Immune suppression | Lack of response to diet change/antimicrobial therapy or confirmed IBD on histopathology | 2-4 mg/kg/day for 2-3 weeks then tapered by 25-50% every 2-4 weeks until lowest effective dose controlling symptoms is achieved | PU/PD Polyphagia Cardiomyopathy Infections |
| Methylprednisolone | Immune suppression | Alternative for patients that refuse oral medication | 10 mg/kg SC every 2-4 weeks, tapered to every 4-8 weeks | As above Diabetes mellitus |
| Chlorambucil | Alkylating agent | ScLSA or refractory cases of IBD | Cats > 4 kg: 2 mg PO Q48 h Cats < 4 kg: 2 mg PO Q72 h |
Bone marrow suppression Neurotoxicity |
| Cyclosporine | Inhibits T cell function | Severe or refractory cases of IBD | 5 mg/kg PO Q12-24 h | Vomiting, diarrhea, hepatopathy |
| Azathioprine | Interferes with DNA synthesis |
Severe or refractory cases of IBD | 0.3 mg/kg PO Q48 h | Severe bone marrow suppression |
| Metronidazole | Anaerobic activity Possible immunomodulatory properties |
Severe or refractory cases of IBD | 10-15 mg/kg/day PO SID (25 mg/kg/day if using metronidazole benzoate) | Neurotoxicity with chronic use |
| Cobalamin (B12) | Cofactor for methylation | Cobalamin levels < 300 ng/L | 250 mg SC/cat once a week for 6 weeks, then 1 dose after 30 days and retesting after 30 days. Continue monthly injection if levels within normal range. |
None reported |
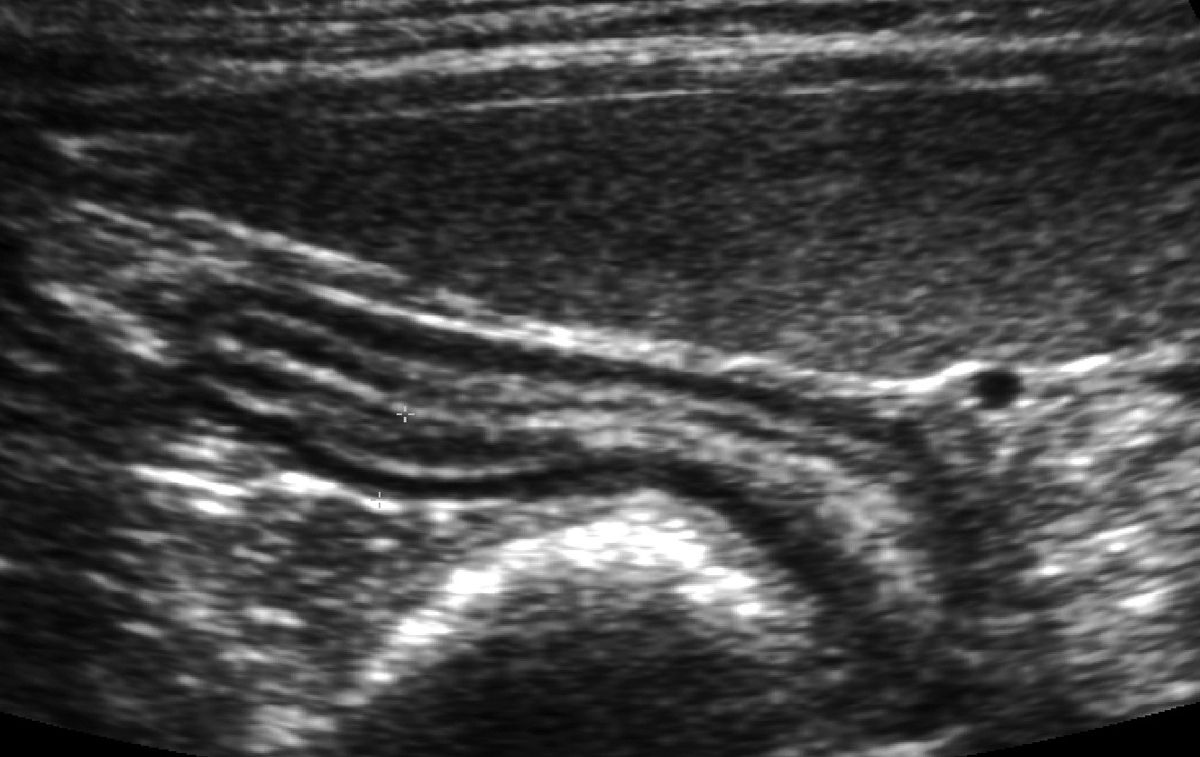
In these more serious cases, an abdominal ultrasound study may reveal findings consistent with small intestinal disease, although thickened intestines or enlarged abdominal lymph nodes can be non-specific (Figure 5). The character and distribution of thickened intestinal walls may influence my recommendation of endoscopy or surgical biopsy, and a single focal thickening might heighten my suspicion for an intestinal adenocarcinoma. Ultrasound can also be a useful modality for looking for extraintestinal disease (Figure 6) but like any other diagnostic test, it is most effective when it follows clinical judgment – an ultrasound exam should not be a “fishing trip”. Whether biopsy tissue is best obtained by endoscopy (partial thickness, limited access) as in Figure 7 or laparotomy (full thickness, unlimited access) is the focus of a number of recent publications, much historical debate, and is not a simple question to answer. Regardless of how I obtain the tissue, I first check with my diagnostic laboratory to ensure I prepare the samples in a way that will allow me to make full use of the diagnostic testing available (e.g., special media). I ask the pathologist to interpret the histopathology using WSAVA guidelines, reporting on cell type, severity, and architectural changes. I take full advantage of advanced diagnostic techniques, including immunohistochemistry, flow cytometry, and PCR, to help determine cell phenotype and look for clonality [22].
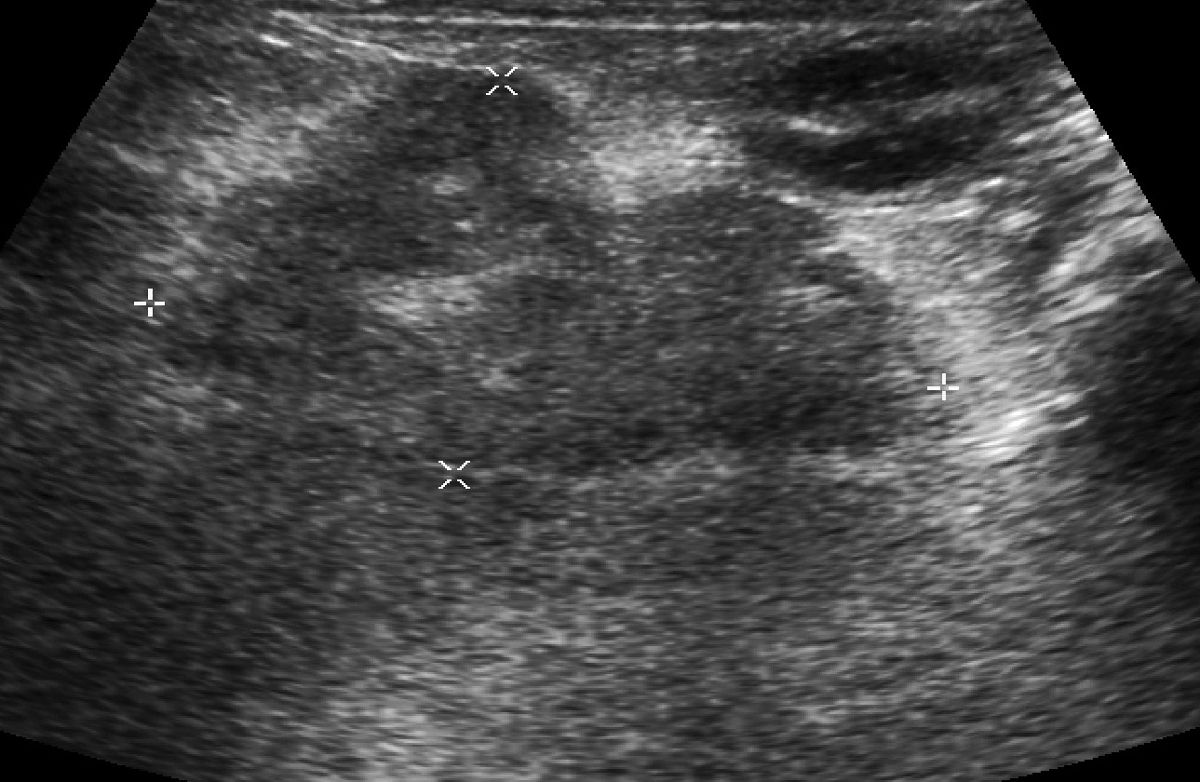
If the histopathology and molecular assay results match my Script Recognition and Clinical Reasoning, I proceed with treatment. If it does not, I restart my “cine loop” and try to make sense of the incongruity.
My preferred treatment for both feline IBD and lymphoma are as set out in the “Chronic enteropathy in cats” paper although I like to keep track of the number of medications I am asking the owner to give their sick cat, and avoid polypharmacy if at all possible.
In summary, I approach a cat with chronic diarrhea as a clinician, first and foremost. That is what I am trained to be, and that is what the client is paying me to do. Fortunately, that approach is also the best way for me to find an effective path to a correct diagnosis and an effective treatment.
Craig B. Webb
PhD, Dr Vétérinaire, Dipl. ACVIM
États-Unis
Craig Webb est actuellement Professeur de médecine des animaux de compagnie et Directeur intérimaire de l'hôpital du CSU. Diplômé de l'Université Madison du Wisconsin, il a fait un internat au Alameda East Veterinary Hospital et un résidanat en médecine des petits animaux au CSU avant d'obtenir son doctorat en neurosciences à l’Université Hahnemann de Philadelphie. Son expertise clinique est centrée sur la gastro-entérologie et l'endocrinologie. Il a été récompensé par le prix Zoetis du « Distinguished Veterinary Teacher » en 2013 et élu membre exceptionnel du corps professoral de la Colorado Veterinary Medical Association en 2014.
References
- Canfield PJ, Malik R. Think about how you think about cases. J Feline Med Surg 2016;18:4-6.
- Weese JS, Martin H. Assessment of commercial probiotic bacterial contents and label accuracy. Can Vet J 2011;52:43-46.
- Rijsman LH, Monkelbaan JF, Kusters JG. Clinical consequences of PCR based diagnosis of intestinal parasitic infections. J Gastroenterol Hepatol 2016;doi: 10.1111/jgh.13412 [Epub ahead of print].
- Norsworthy GD, Estep JS, Kiupel M, et al. Diagnosis of chronic small bowel disease in cats: 100 cases (2008-2012). J Am Vet Med Assoc 2013;15:1455-1461.
- Norsworthy GD, Estep JS, Hollinger C, et al. Prevalence and underlying causes of histologic abnormalities in cats suspected to have chronic small bowel disease: 300 cases (2008-2013). J Am Vet Med Assoc 2015;247:629-635.
- Guilford WG, Markwell PJ, Jones BR, et al. Prevalence and causes of food sensitivity in cats with chronic pruritus, vomiting or diarrhea. J Nutr 1998;128:2790S-2791S.
- Guilford WG, Jones BR, Markwell PJ, et al. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med 2001;15:7-13.
- Mandigers PG, Biourge V, van den Ingh TS, et al. A randomized, open-label, positively controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy. J Vet Intern Med 2010;24:1350-1357.
- Simpson JW. Diet and large intestinal disease in dogs and cats. J Nutr 1998;128:2717S-2722S.
- Freiche V, Houston D, Weese H, et al. Uncontrolled study assessing the impact of a psyllium-enriched extruded dry diet on faecal consistency in cats with constipation. J Feline Med Surg 2011;13:903-911.
- Steiner JM. Exocrine pancreatic insufficiency in the cat. Top Companion Anim Med 2012;27:113-116.
- Marks SL. Rational approach to diagnosing and managing infectious causes of diarrhea in kittens. In: Little SE (ed). August’s Consultations in Feline Internal Medicine. Vol. 7. Philadelphia: Elsevier, 2016;1-22.
- Maunder CL, Day MJ, Hibbert A, et al. Serum cobalamin concentrations in cats with gastrointestinal signs: correlation with histopathological findings and duration of clinical signs. J Feline Med Surg 2012;14:689-693.
- Kiselow MA, Rassnick KM, McDonough SP, et al. Outcome of cats withlow-grade lymphocytic lymphoma: 41 cases (1995-2005). J Am Vet MedAssoc 2008;232:405-410.
- Sabattini S, Bottero E, Turba ME, et al. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J Small Anim Pract 2016;57:396-401.
- Marks SL, Rankin SC, Byrne BA, et al. Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. J Vet Intern Med 2011;25:1195-1208.
- Laflamme DP, Xu H, Cupp CJ, et al. Evaluation of canned therapeutic diets for the management of cats with naturally occurring chronic diarrhea. J Feline Med Surg 2012;14:669-677.
- Zoran DL. Nutritional management of feline gastrointestinal diseases. Top Compan Anim Med 2008;23:200-206.
- Leib MS. Treatment of chronic idiopathic large-bowel diarrhea in dogs with a highly digestible diet and soluble fiber: a retrospective review of 37 cases. J Vet Intern Med 2000;14:27-32.
- Bybee SN, Scorza AV, Lappin MR. Effect of the probiotic Enterococcus faeciuim SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J Vet Intern Med 2011;25:856-860.
- Gookin JL, Copple CN, Papich MG, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med 2006;20:536-543.
- Lalor SL, Gunn-Moore DA. Effects of concurrent ronidazole and probiotic therapy in cats with Tritrichomonas foetus-associated diarrhea. J Feline Med Surg 2012;14:650-658.
Other articles in this issue
Share on social media