How I approach... The dog with altered hepatic enzymes
Written by Jordi Puig
Elevated liver enzymes on routine biochemistry screens are a daily occurrence in small animal practice; Jordi Puig discusses how he decides if such findings are significant or not.
Article
Key Points
Liver enzyme levels do not indicate hepatic functionality; this requires evaluating parameters which reflect the liver’s capacity for synthesis and/or excretion of compounds, such as bile acids.
A single measurement does not provide enough information in most cases, and serial monitoring is much more helpful.
The biochemical changes found in patients with a secondary hepatopathy are usually caused by a non-specific reactive hepatitis.
Where there is advanced liver disease, such as cirrhosis, any increase in hepatic enzymes may be slight.
Introduction
The correct diagnosis of a hepatobiliary disease can be a difficult task. An increase in liver enzymes is a common finding in all veterinary practices, and we must comprehend its meaning in order to establish a suitable diagnosis and treatment. Understanding the advantages and disadvantages of diagnostic laboratory testing is essential in order to avoid incorrect interpretation of results.
Basic principles of liver enzymology
Most methods used to measure enzyme levels are based on the calculation of their activity. The enzyme unit (U) is the amount of an enzyme that catalyzes the conversion of one μmol of substrate per minute [1]. Ranges vary between laboratories and methodologies, and when comparing results it must always be in relation to the magnitude of the increase and not simply with absolute numbers. Additionally, remember that hemolysis, jaundice, or lipemia can alter the sample results, depending on the analytical method used.
The magnitude of the increase in enzyme activity tends to be in proportion to the severity of liver damage; however such tests do not predict liver function, the cause of the problem, or the prognosis. For example, where there is advanced illness such as cirrhosis, the increase in liver enzymes may be slight. Likewise, the duration of any increase depends mainly on the average half-life of the enzyme, the cause of the damage, and the severity of the process. Because of this, a single measurement rarely provides enough information for the clinician, and serial monitoring is much more revealing. Any increase in liver enzymes can be graded into 3 stages [2]:
- Mild: < 5 times the upper limit of the reference range
- Moderate: 5-10 times the upper limit of the reference range
- Severe: > 10 times the upper limit of the reference range
The main mechanisms that cause increased serum liver enzymes are cell damage and the induction of enzyme synthesis. The enzymes are mainly found in the hepatocyte mitochondria, cytoplasm, or cell membrane. Where enzymes are elevated due to cell damage, the leaking of enzymes depends on their concentration and location within the cell. For example, an increase in enzymes located in the mitochondria suggests greater damage than an increase in enzymes located only in the cytoplasm. Liver enzymes are generally classified into two groups, those that indicate cell damage (alanine aminotransferase and aspartate aminotransferase) and those that indicate enzyme synthesis (alkaline phosphatase and gamma-glutamyl transferase) [3].
Finally, the measurement of liver enzymes is not proof of functionality. The assessment of liver function is based on evaluating parameters which reflect its capacity for synthesis and/or excretion, such as bilirubin, glucose, cholesterol, urea, albumin, or the bile acid stimulation test (Table 1).
| Liver enzymes do not inform us about the functional capacity of the liver. The most common tests employed to determine hepatic function are: |
|
Table 1. Liver function tests.
Alanine aminotransferase (ALT)
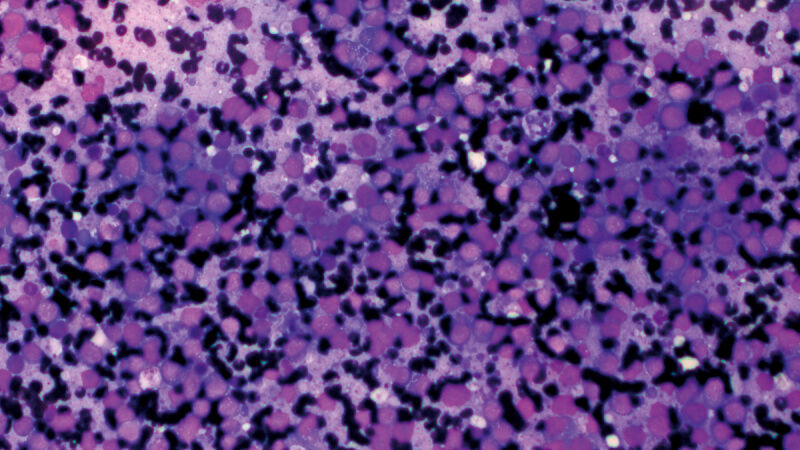
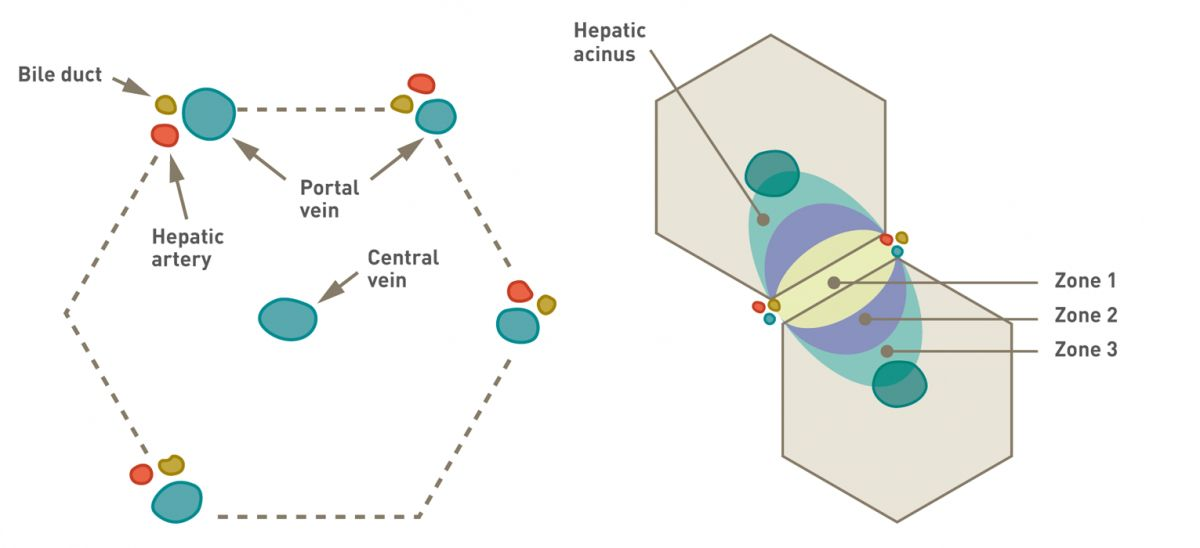
ALT, previously known as serum glutamic pyruvate transaminase (SGPT), is found primarily within the cytoplasm of hepatocytes, with higher levels in zone 1, the periportal area (Box 1) [2]. It is also found in other organs (heart muscle, skeletal muscle, kidneys and red blood cells), but ALT levels in the liver are 4 times higher than in heart muscle and 10 times higher than in the kidney. If an increase in ALT is seen, it is important to exclude a non-liver-related origin (e.g., hemolysis or severe muscle trauma). The average half-life of the enzyme is estimated to be around 2-3 days.
The release of the enzyme is typically associated with alterations in the permeability of the hepatocellular membrane, and is most commonly caused by toxins, inflammatory processes, hypoxia, tissue trauma, or neoplasia (Table 2). The largest increases are seen with necrosis and inflammation. The magnitude of the increase correlates to the degree of cellular damage, but is not specific for any particular process. In advanced cirrhosis or vascular disease it is common to find only slight elevations. It is important to remember that increased ALT is not always synonymous with primary liver disease; many illnesses can result in elevated liver enzymes and the primary cause can be distant to the liver (e.g., metabolic disease, systemic inflammatory processes). In acute disease, a decrease in ALT levels of greater than 50% during the first few days of illness is considered a positive prognostic factor.
|
Aspartate aminotransferase (AST)
AST (which was previously known as serum glutamic oxaloacetic transaminase (SGOT)), is found in the hepatocyte mitochondria at higher concentrations than ALT, and is more predominant in zone 3 of the hepatic acinus (Box 1) [2]. AST has a lower specificity than ALT and can also be found in muscle and red blood cells. Like ALT, it is important to exclude a non-liver-related origin (e.g., from hemolysis or muscle trauma) if elevated levels are detected, but the differential diagnosis is similar to that of ALT. The half-life of AST in the dog is 5 to 12 hours. In most cases the increase in AST and ALT activity is in parallel, but in some patients AST will normalize before ALT due to its shorter half-life and its mitochondrial location.
Alkaline phosphatase (AP)
AP is codified by two genes: a non-specific tissue gene and an intestinal gene. The non-specific tissue gene transcribes the isoenzymes found in the liver, kidney, placenta, and bone [2]; the intestinal gene codes for the intestinal and corticosteroid-induced isoenzymes. The isoenzymes catalyze the same chemical reaction but have a different sequence of amino acids. The half-life of intestinal, kidney, and placenta AP is very short (less than 6 minutes). However, the half-life of AP in the liver and bone and corticosteroid-induced AP is almost 60 hours. In animals under a year old, bone-derived AP constitutes the majority of the total AP [5]. In older animals the liver isoenzyme predominates. Corticosteroid-induced AP contributes 10-30% of the total AP level, with the higher percentage in older dogs. Because of this,, the specificity of the enzyme is around 51% for hepatobiliary diseases, but its sensitivity is 80% (Table 3) (Box 2).
|
|
Liver-derived AP is located in the hepatocyte membrane of the bile canaliculi and sinusoids. The two main mechanisms responsible for an increase in hepatic AP are cholestasis and drug induction. Cholestasis produces an accumulation of bile acids, which induces the production of AP. Drugs such as phenobarbital and corticosteroids will increase hepatic AP.
Corticosteroid-induced AP is produced in the liver. Levels tends to be increased with hyperadrenocorticism, but the isoenzyme can also be elevated with other conditions such as diabetes mellitus, primary liver disease, or other chronic processes; this limits its use in the diagnosis of hyperadrenocorticism.
Bone-specific AP is located in the membrane of osteoblasts. Increases in osteosarcoma cases tend to be slight. A benign familial hyperphosphatasemia (with a rise in mainly bone-specific AP) has been described in Siberian Huskies [5].
The most notable elevations in AP can be seen in cholestasis (focal or diffuse), hepatitis, or with corticosteroid use. Certain liver tumors, such as hepatocellular carcinomas, can also cause a significant increase. AP activity levels do not help to distinguish between hepatic cholestasis and post-hepatic cholestasis (Table 3).
Gamma-glutamyl transferase (GGT)
GGT is an enzyme found in epithelial cells of the biliary system and hepatocytes. It is also present in the pancreas, kidney tubules, and epithelial cells of mammary tissue. The half-life in a dog is 72 hours. Elevated GGT is related to cholestasis or biliary hyperplasia, but corticosteroids will also increase its activity. The enzyme is considered to be more specific (87%) than AP but less sensitive (50%) [3].
Approaching the patient with increased liver enzymes
My main objectives when a hepatobiliary disease is suspected, based on biochemistry screens, are as follows:
- Determine if there is a hepatobiliary disease.
- Evaluate liver function.
- Determine if the origin is primary or secondary.
- Establish the correct diagnosis.
- Monitor the response to treatment.
Although these seem clear in principle, altered liver enzymes are a challenge as clinical signs can be very unspecific and in some cases absent. Furthermore, the liver plays a major role in detoxifying both endogenous and exogenous toxins, and there are many extrahepatic processes which affect it on a secondary level. The liver has a high-reserve capacity and signs of liver dysfunction are only seen in advanced disease processes (Figure 1).
The first step requires integration of the history, clinical signs, and physical examination. It is of utmost importance to obtain an optimal history to identify any possible toxin (diet, drugs, plants, etc.) and determine if there are risk factors for infectious disease (e.g., a poor vaccination schedule). Due to its anatomo-functional situation and its ability to metabolize foreign compounds (xenobiotics), the liver may be exposed to high concentrations of substances which can have toxic effects [6]. It is also well recognized that some dog breeds are predisposed to certain liver problems.
Hepatotoxicity due to drugs can be divided into two groups: intrinsic and idiosyncratic. The former causes liver damage in any animal exposed to a given dose of a drug; the latter occurs in individual animals where the liver damage is unpredictable and with no apparent correlation to the drug dosage.
- NSAIDs have been associated with idiosyncratic reactions. Most reported cases have been due to carprofen, although all NSAIDs are capable of causing damage. The severity depends on the patient and is typically seen within three weeks of starting the drug. The signs are variable and the increase in liver enzymes severe (especially ALT). Although most dogs recover when the NSAID is withdrawn and given supportive therapy, some can die due to acute liver failure.
- The toxic dose of paracetamol in dogs is around 150 mg/kg and is an example of intrinsic hepatotoxicity. The metabolites of this drug (mainly N-acetyl-p-benzoquinone imine, or NAPBQ) are involved in oxidation of red blood cells and hepatocytes. Laboratory findings include methemoglobinemia, a noticeable increase in ALT, and hyperbilirubinemia in most affected dogs [7]. Intravenous acetylcysteine is the treatment of choice for these cases, as it reduces the toxicity of NAPBQ. Cimetidine, vitamin C and S-adenosylmethionine (SAMe) can also help.
- The hepatotoxicity of phenobarbital has been described as both intrinsic and idiosyncratic. Although the most commonly stated theory to explain the increase in AP is induction, this is debatable; liver damage may also occur [8] [9]. Basically, increases in AP are slight and in some cases there is a mild elevation of ALT. The indications to withdraw phenobarbital treatment include when ALT levels are higher than AP and/or there is evidence of liver dysfunction (hypocholesterolemia, hyperbilirubinemia, increase in bile acids, or hypoalbuminemia). Although clinical and histopathological changes may be severe, some patients can recover their liver function [10].
- Azathioprine is a purine analog widely used to treat immune-mediated disorders. Its ability to cause hepatotoxicity (defined as > 2 times the increase in ALT activity) has been described in 15% of treated dogs, generally without clinical signs [11]. Hepatotoxicity normally occurs during the first two weeks of therapy, before myelotoxicity (which on average can take 53 days to develop) and it is suspected that German Shepherd dogs are predisposed to it. However, in some cases, the enzyme increase occurs later. Where there is a slight elevation in ALT, it is wise to monitor the patient regularly, but for moderate or severe increases, it is recommended to reduce the dose or stop the medication.
- The increase of AP in dogs treated with glucocorticoids is variable and normally not associated with liver damage. However, in some cases, the appearance of a severe vacuolar hepatopathy may cause signs of cholestasis and cell damage. The effects are minimized by reducing the dose, although full remission may take months. It is not unusual to see slightly elevated bile acids in patients receiving glucocorticoids in the absence of liver disease. Administering liver protectants such as SAMe does not influence either the histopathology or the altered enzyme levels in animals on glucocorticoids [12].
- Lomustine (CCNU) is an alkylating compound with hepatotoxicity reported in 6% of treated dogs. The toxic effects have been described as delayed in the course of treatment, accumulative, dose-dependent, and irreversible. Enzyme alteration ranges from moderate-to-severe ALT, with an average of 11 times the upper limit of the reference range. The prognosis is serious due to liver failure, but administration of SAMe and silibinin during treatment may help minimize liver damage [13].
What other tests can be performed?
In dogs with increased liver enzymes, I always obtain a complete blood count (CBC) and biochemistry profile, and urinalysis. Findings from the blood count can be quite variable. If anemia is present, this is usually non-regenerative, although intestinal bleeding secondary to a coagulopathy can also occur. Microcytosis is frequently seen with portosystemic shunts. In patients with liver failure or portosystemic shunts, it is common to find ammonium biurate crystals in the urine sediment.
The magnitude of the increase in hepatic enzyme activity tends to be in proportion to the severity of liver damage; however it does not predict function, the cause of the process, or the prognosis.
Radiography can help determine the size, shape, position, opacity, and edges of the liver, and will also detect the presence of any gas or mineralization (Figure 2). Ultrasound helps to determine the extent of hepatic injury (focal, multifocal, or diffuse), as well as evaluation of vascularization, and can aid sampling (for cytology, culture, and biopsy) (Figure 3). Remember that the absence of ultrasound changes is not synonymous with a healthy liver.
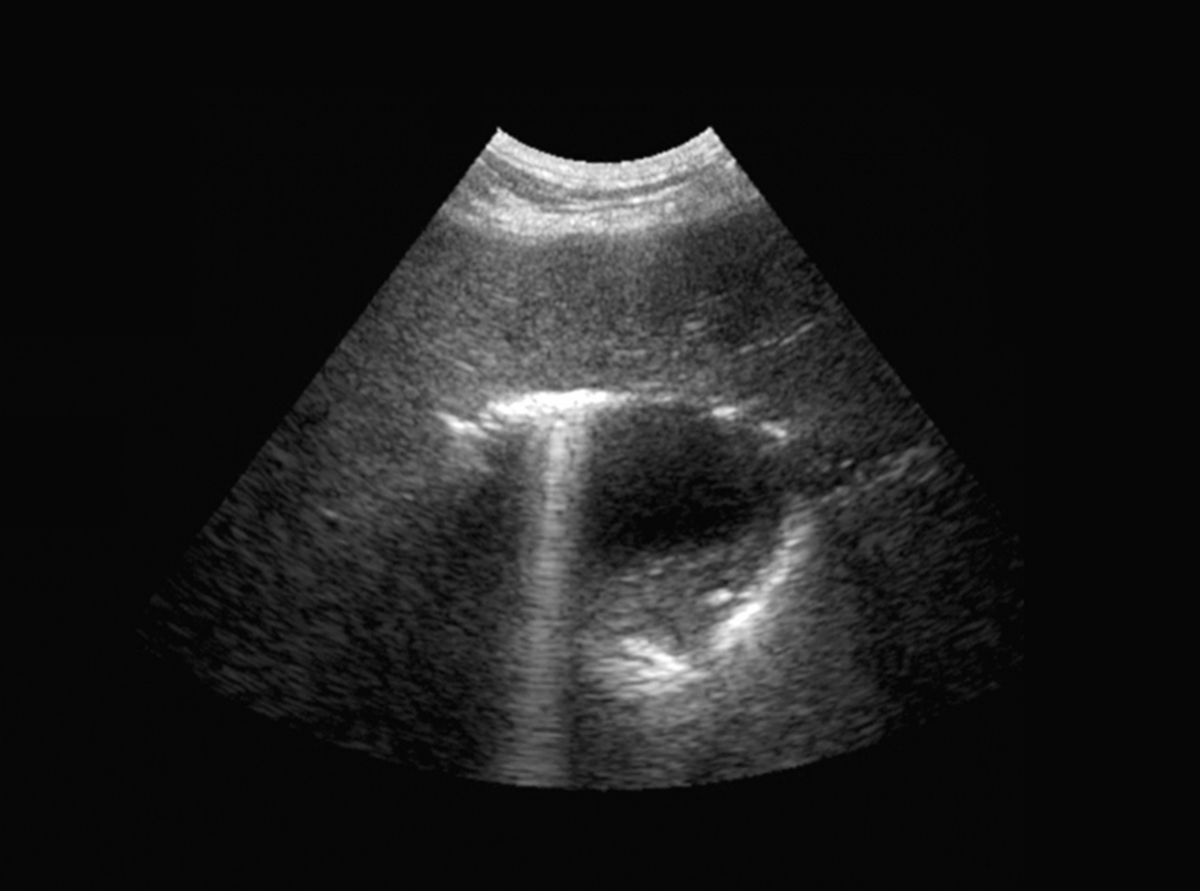
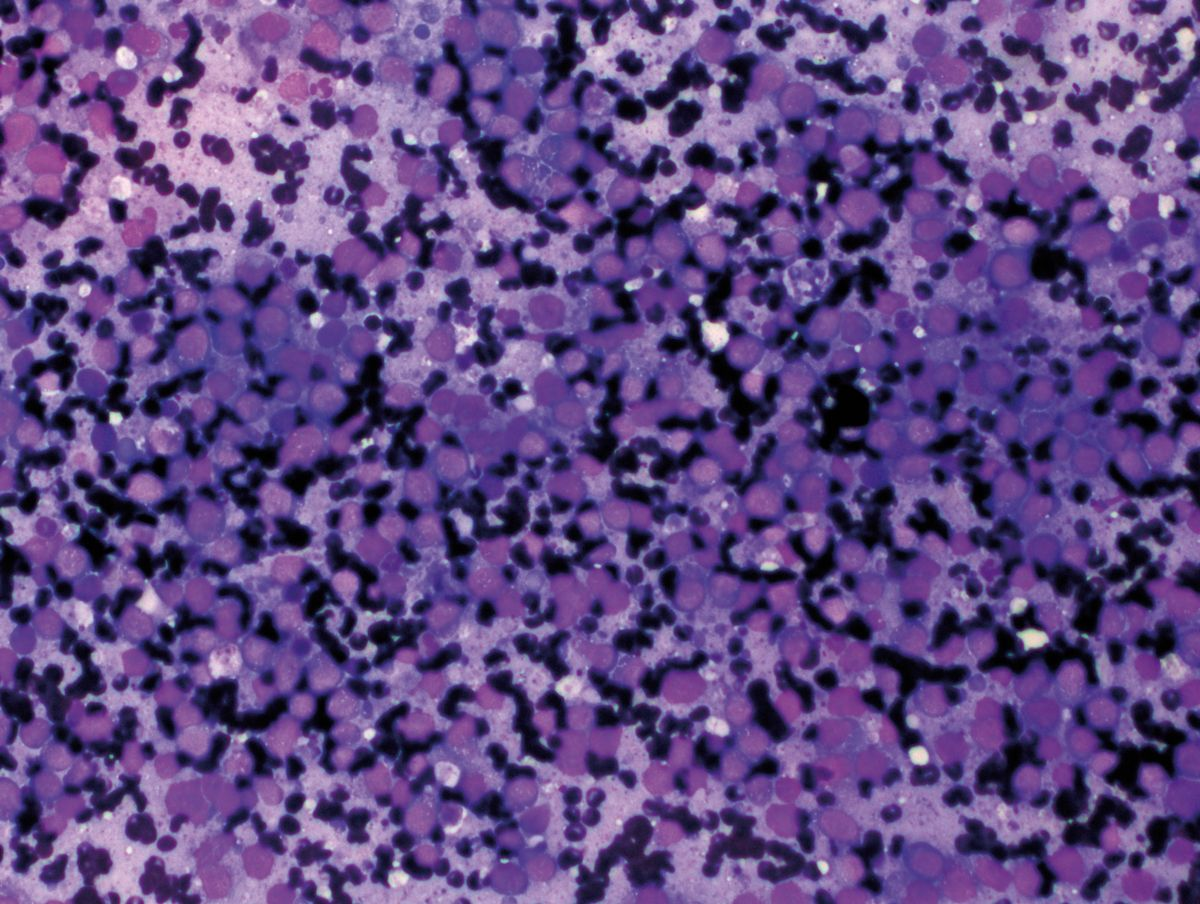
Liver cytology is mainly of value when dealing with multifocal or diffuse metabolic or neoplastic processes (e.g., round cell tumor, vacuolar hepatopathies) (Figure 4). However, sensitivity is low when compared with histopathology, but since the process is fast, minimally invasive, and safe, I recommend it in many cases as a first step for taking liver samples. Ultrasound-guided cholecystocentesis is another useful minimally invasive test, with few associated complications [14].
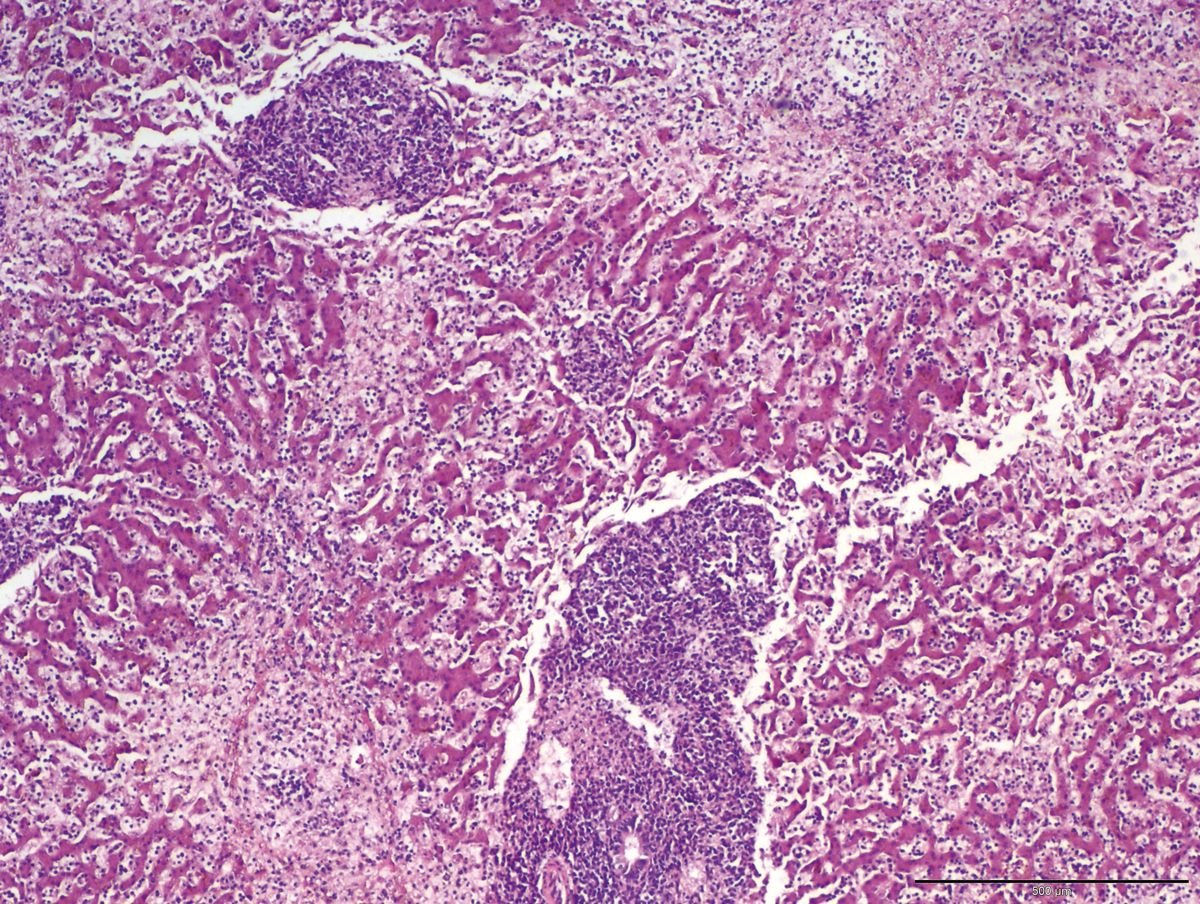
Histopathology is necessary to distinguish malignant and benign neoplasia, identify vascular abnormalities (portal vein hypoplasia), cirrhosis, inflammatory processes, or liver disease due to copper accumulation or other metals/substances (Figure 5). Following coagulation tests, it is essential to always obtain multiple samples of the liver lobes, and various methods (Tru-Cut©, laparotomy, or laparoscopy) may be employed. It is extremely important that the pathologist follows the World Small Animal Veterinary Association (WSAVA) guidelines for liver histopathology1 when interpreting the samples.
1 wsava.org/global-guidelines/liver-disease-guidelines
Hyperbilirubinemia
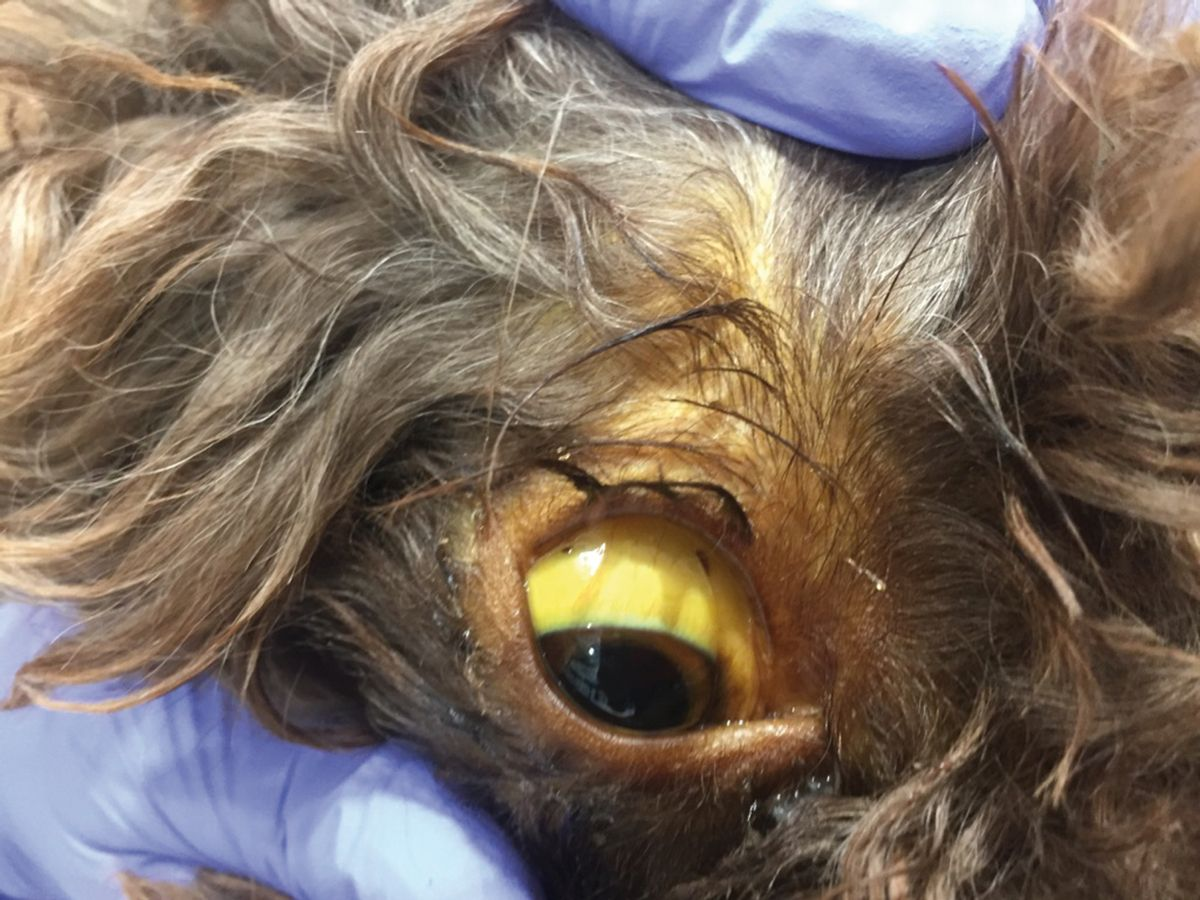
When presented with a jaundiced dog, it is crucial to determine the origin of the hyperbilirubinemia (pre-, post-, or hepatic) via blood sampling and ultrasound imaging (Figure 6). Recent studies have shown that bacterial cholangitis and cholecystitis in dogs are possibly more common than was previously thought [15]. The most typical clinicopathological findings are an increase in liver enzymes, hyperbilirubinemia, and neutrophilia. The most frequent ultrasound findings are distension of the bile duct, thickening of the gallbladder wall, distension of the gallbladder, and the presence of bile sediment or mucocele. Bile sampling is important to check for potential antibiotic resistance, and the preferred treatment for the condition is usually cholecystectomy, which also allows for biopsies/cultures to be obtained. Other common conditions of the gallbladder and bile ducts are mucocele, cholelithiasis, and neoplasia.
Secondary liver disease
It is possible that the most difficult part of approaching a patient with increased liver enzymes is distinguishing between primary or secondary liver disease. The changes found in patients with secondary liver disease are typically due to a non-specific reactive hepatitis. Most cases show increased enzyme levels compatible with cell damage (ALT and AST) and enzyme induction (AP and GGT). However, altered liver function is rare except with functional cholestasis. Biopsy will demonstrate an inflammatory infiltrate in the portal areas and parenchyma, without signs of liver necrosis. Other changes can also be seen, such as vacuolar degeneration, lipidosis, or cholestasis, the latter being the most common histopathological finding in biopsies. Patients with primary liver disease are more likely to have more serious clinical signs, such as hepatomegaly, microhepatica, jaundice, or hepatic encephalopathy.
It is possible that the most difficult part of approaching a patient with increased liver enzymes is distinguishing between primary or secondary liver disease. The changes found in patients with secondary liver disease are typically due to a non-specific reactive hepatitis.
Chronic hepatitis
Chronic hepatitis often presents in dogs with vague clinical signs and increased liver enzymes. At the histopathological level, it is characterized by apoptosis or necrosis associated with an inflammatory infiltrate (mixed or lymphoplasmacytic) which tends to progress to fibrosis and cirrhosis with liver failure. The etiology is diverse (copper storage disease, infectious agents, drugs, etc.) although in many cases the cause is unknown (idiopathic chronic hepatitis). Some breeds are predisposed to chronic hepatitis; the most studied ones are those prone to copper storage hepatopathy. It is important to remember that to quantify the amount of hepatic copper, a large sample (1-2 grams) of liver biopsy tissue is required. Various infectious agents can also cause chronic hepatitis, including Leptospira, Leishmania, Babesia, and Ehrlichia spp. The most common histopathological finding in animals with leishmaniasis is a granulomatous inflammation or multifocal pyogranulomatous inflammation in the hepatic portal areas.
What about the asymptomatic patient?
It is common to detect elevated liver enzymes in an asymptomatic patient; one study in a group of healthy dogs of differing ages revealed significant numbers of animals had increased levels of ALT, AST, AP, and/or GGT, at 17%, 11%, 39%, and 19% respectively [16]. My first step in this situation is to confirm the results (by repetition of the tests or by obtaining a second sample, avoiding hemolysis or lipemia) to exclude laboratory error. Obtaining a clinical history is important in order to detect causes such as drug administration (including topical treatments or drops) or signs not recognized by the owners beforehand. Age is important; young animals can show mild increases in AP, and in older animals enzyme increases tend to be compatible with benign processes (nodular hyperplasia), neoplasia, or vacuolar hepatopathies. One of the most important steps is to determine the origin of the enzyme change, since in many cases the primary pathology is distant to the liver. Diagnosis and resolution of the primary cause will often result in normalization of the enzymes; for example, 50% of dogs with tracheal collapse have increased liver enzymes and bile acids, possibly due to hepatic hypoxia. Although treating the respiratory problem reduces the bile acid levels, hepatic enzymes tend to remain high [17].
An increase in AP detected during an annual check-up or a pre-anesthetic analysis is a common finding. Since increased AP could be due to an isoenzyme, obtaining the clinical history must be exhaustive. The most common endocrine diseases associated with an increase in AP are diabetes, hyperadrenocorticism, and hypothyroidism. 90% of hyperadrenocorticism cases have increased AP, due to enzyme induction and vacuolation of the hepatocytes with glycogen, causing cholestasis. In diabetes mellitus, there is vacuolation of hepatocytes with lipidosis and cholestasis. As noted above, the most common causes of increased AP in asymptomatic older dogs are vacuolar hepatopathy, nodular hyperplasia, or neoplasia.
Vacuolar hepatopathy may be linked to endogenous or exogenous corticosteroids, and this can sometimes be severe, with cholestasis and cell damage leading to an increase in ALT [18]. In 50% of the cases described, there is no evidence of adrenal disease or exogenous administration of corticosteroids, and the exact cause is unknown.
Nodular hyperplasia is characterized by multiple nodules in the liver parenchyma and is a benign condition in older dogs. The etiology is unknown, but the WSAVA categorization of liver disease classifies it as a neoplastic process. It is important to distinguish hyperplasia from tumor-related processes or cirrhosis. The increase in AP may be accompanied by slight increases in ALT, but liver function in these cases is normal. There is no specific treatment, although a biochemistry check-up and regular ultrasound every 6-12 months is recommended.
Elevated hepatic enzymes are a common finding in small animal practice but such results do not tell us about the functional capacity of the patient’s liver. There are numerous causes for such findings, and the clinician must take into account other diagnostic tests, the patient’s history and the clinical signs to make an appropriate diagnosis and thus enable correct treatment.
References
- Stockham SL, Scott MA. Enzymes. In; Fundamentals of Veterinary Clinical Pathology. Iowa; John Wiley & Sons 2008;639-674.
- Dayrell-Hart B, Steinberg SA, Van Winkle TJ, et al. Hepatotoxicity of phenobarbital in dogs: 18 cases (1985-1989). J Am Vet Med Assoc 1991;199(8):1060-1066.
- Wallisch K, Trepanier LA. Incidence, timing, and risk factors of azathioprine hepatotoxicosis in dogs. J Vet Intern Med 2015;29(2):513-518.
- Center SA, Warner KL, McCabe J, et al. Evaluation of the influence of S-adenosylmethionine on systemic and hepatic effects of prednisolone in dogs. Am J Vet Res 2005;66(2):330-341.
- Skorupski KA, Hammond GM, Irish AM, et al. Prospective randomized clinical trial assessing the efficacy of Denamarin for prevention of CCNU-induced hepatopathy in tumor-bearing dogs. J Vet Intern Med 2011;25(4):838-845.
- Policelli Smith R, Gookin JL, Smolski W, et al. Association between gallbladder ultrasound findings and bacterial culture of bile in 70 cats and 202 dogs. J Vet Intern Med 2017;31(5):1451-1458.
- Tamborini A, Jahns H, McAllister H, et al. Bacterial cholangitis, cholecystitis, or both in dogs. J Vet Intern Med 2016;30(4):1046-1055.
- Comazzi S, Pieralisi C, Bertazzolo W. Haematological and biochemical abnormalities in canine blood: frequency and associations in 1022 samples. J Small Anim Pract 2004;45(7):343-349.
- Bauer NB, Schneider MA, Neiger R, et al. Liver disease in dogs with tracheal collapse. J Vet Intern Med 2006;20(4):845-849.
- Sepesy LM, Center SA, Randolph JF, et al. Vacuolar hepatopathy in dogs: 336 cases (1993-2005). J Am Vet Med Assoc 2006;229(2):246-252.
- Center SA. Interpretation of liver enzymes. Vet Clin North Am Small Anim Pract 2007;37(2):297-333.
- Chapman SE, Hostutler RA. A laboratory diagnostic approach to hepatobiliary disease in small animals. Vet Clin North Am Small Anim Pract 2013;43(6):1209-1225.
- Toulza O, Center SA, Brooks MB, et al. Evaluation of plasma protein C activity for detection of hepatobiliary disease and portosystemic shunting in dogs. J Am Vet Med Assoc 2006;229(11):1761-1771.
- Fernandez NJ, Kidney BA. Alkaline phosphatase: beyond the liver. Vet Clin Pathol 2007;36(3):223-233.
- Weingarten MA, Sande AA. Acute liver failure in dogs and cats. J Vet Emerg Crit Care (San Antonio) 2015;25(4):455-473.
- Stewart JE, Haslam AK, Puig J. Pathology in practice; acetaminophen (paracetamol) toxicosis. J Am Vet Med Assoc 2016;248(9):1009-1011.
- Müller PB, Taboada J, Hosgood G, et al. Effects of long-term phenobarbital treatment on the liver in dogs. J Vet Intern Med 2000;14(2):165-171.
- Gaskill CL, Miller LM, Mattoon JS, et al. Liver histopathology and liver and serum alanine aminotransferase and alkaline phosphatase activities in epileptic dogs receiving phenobarbital. Vet Pathol 2005;42(2):147-160.
Jordi Puig
DVM, Dipl. ACVIM (SAIM), Dipl. ECVIM-CA (Internal Medicine)
Spain
Dr. Puig graduated from the Autonomous University of Barcelona in 2008 and after a short period in general practice undertook an internship and then a residency at the Animal Health Trust in the United Kingdom. He gained his Diploma from the American College of Veterinary Internal Medicine (Small Animal Internal Medicine) in 2014 and the European College of Veterinary Internal Medicine – Companion Animals (Internal Medicine) in 2017. He joined Ars Veterinary Hospital in 2015, where he is currently head of the Small Animal Internal Medicine Department. He is interested in all aspects of internal medicine, with a research focus on gastroenterology and endocrinology.
Other articles in this issue
Share on social media