Perianal pruritus in the dog
Written by Elisa Maina and Chiara Noli
Anal and perianal pruritus can be a real nuisance and requires careful diagnosis as there are several etiologies.

Key points
Perianal pruritus may be defined as itchiness in the region around the anus, ranging from the ventral tail base to (but excluding) the genitalia.
Typical presentations of perianal pruritus are scooting, licking or chewing at the anal/perianal region and/or under the tail. Secondary signs are common and may include erythema, excoriations, alopecia, hyperpigmentation and lichenification.
There are various etiologies of perianal pruritus, including inflammatory (mainly allergic), parasitic, infectious and neoplastic diseases; a diagnosis requires a methodical approach, as successful identification and treatment of the underlying cause is essential in order to effect a cure.
Introduction
Perianal pruritus, or pruritus ani, has been recently defined as “itchiness in the region around the anus, ranging from the ventral tail base to (but excluding) the genitalia” [1]. Dogs try to alleviate their discomfort by “scooting” along the floor, and/or by licking or chewing the area. Despite the fact that it is a common problem in practice, there has been little in the way of research; indeed, only one published study to date specifically considers canine perianal pruritus [1], and notes that 37% of dogs presented to a dermatological specialist had this clinical sign. The aim of this review is to address the etiologies of the condition, discuss the diagnostic work-up, and review current medical treatments.
Etiology
Perianal pruritus is not found in healthy dogs [2], and may be the result of a number of different causes. These can be broadly subdivided into non-dermatological and dermatological problems, and each is discussed briefly below.
Non-dermatological causes
Intestinal parasites
Intestinal parasites of dogs have a worldwide distribution, although the prevalence varies geographically from 12.5-34.4% [3]. Whilst deworming is regularly done for puppies, it is less common in adults. The most common intestinal parasites are roundworms, hookworms, whipworms and tapeworms; of these, only whipworms and tapeworms have been associated with anal pruritus [3].
Trichuris vulpis is a whipworm commonly found in dogs. The life cycle is direct, with transmission being via ingestion of the eggs, followed by larval migration to the cecum and colon where they penetrate into the mucosa and mature. Eggs are laid into the gut lumen and released into the environment via the feces. Clinical signs depend on the degree of infestation, the presence of other diseases and the nutritional condition of the dog. Although chronic diarrhea is the most important clinical sign, some dogs scoot or lick the perianal area [4].
Dipylidium caninum is a tapeworm distributed worldwide. It has an indirect life cycle, with fleas as the intermediate host. The dog is the definitive host and is infected by ingestion of the adult flea containing the cysticercoid. These worms reside in the small intestine and produce proglottids, which contain eggs. Gravid proglottids may pass intact via the feces or leave the host spontaneously, emerging from the anus and crawling onto the perianal skin; this migration can cause pruritus. The life cycle is continued by ingestion of the eggs by a flea.
Anal sac disease
Anal sacs are cutaneous diverticula of the anus, lined by keratinized, stratified squamous epithelium. Apocrine anal sac glands secrete a mixture of fatty and serous materials and cellular debris; the secretion may vary in amount, color and consistency [1],[5]. Perianal pruritus can be associated with anal sac disease (ASD) [2]; dogs scoot, lick and chew the perianal area to alleviate the discomfort caused by distension of the anal sacs and/or irritation secondary to inflammation or infection. The anal sacs may be affected by the following conditions:
- Impaction: One study documented anal sac impaction in 2.1% of all dogs presenting in small animal practice [6]. Although the exact etiology is unknown, over-secretion or changes in secretion consistency may make passive emptying of the sacs more difficult [7]. Furthermore, changes in muscle tone due to aging or obesity, or even the presence of soft feces, may cause the sacs to overfill [8].
- Infection: Anal sac infection can occur as a consequence of chronic fecal impaction or contamination, incomplete emptying of the colon, obesity, chronic bowel disease, allergy, endocrinopathies, and iatrogenic damage when the sacs are expressed. Infection is characterized cytologically by the presence of inflammatory cells as well as bacteria or yeasts [9], but the presence of bacteria and neutrophils within anal sac contents does not always indicate infection, as they can also be found in healthy dogs [2]. Indeed, dogs with pyoderma but no ASD have much higher levels of intracellular bacteria and inflammatory cells in their anal sacs than dogs with ASD [5].
- Abscessation: Abscesses are well circumscribed masses containing suppurative exudate (Figure 1) that can develop as a consequence of impaction and infection. However, rupture of the abscess can lead to exudate spreading into the surrounding tissue, causing cellulitis and pain, or formation of a perianal fistula.
- Neoplasia: Adenocarcinoma is the most common neoplasm affecting the anal sacs, and is often accompanied by hypercalcemia. Although older females were once believed to be over-represented, this is now questionable, and at least one study which looked at apocrine gland carcinoma of the anal sac in dogs reported an equal gender distribution [10]. Squamous cell carcinoma [11] and malignant melanoma [12] have also been described.
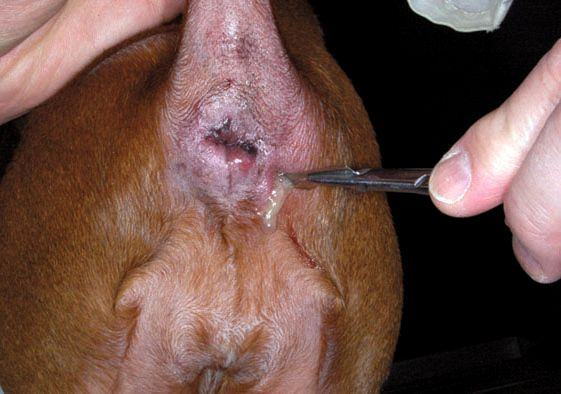
Perianal diseases
- Perianal furunculosis: Also known as perianal fistulae, this is a chronic, debilitating, painful and progressive condition affecting the anus, peri-rectal tissues and perianal skin characterized by inflammation, ulceration and sinus tract formation (Figure 2). The etiology of the disease is still unknown, but an immune-mediated process has been suggested, although since the disease mainly affects German Shepherd dogs there may be a genetic predisposition. Affected dogs may show significant anal discomfort, manifested as pain, tenesmus and licking; hemopurulent discharge may leak from the fistulae. Although furunculosis is not typically considered a primarily pruritic disease, in the initial phases sometimes scooting may be the only presenting sign.
- Neoplasia: Hepatoid glands, also called circumanal or perianal glands, are modified sebaceous glands present in the perianal region; hepatoid gland adenoma is a common neoplasm, accounting for 8-10% of all canine skin tumors [13]. It is especially common in aged intact male dogs (Figure 3) and whilst the etiology is unknown, testosterone may be involved in the development of the condition. Perianal gland carcinoma (including perianal gland epitheliomas) [14] is by comparison rare in the dog. Adenomas and well-differentiated carcinomas are characterized by nodules around the anus; poorly differentiated carcinomas are not well circumscribed and often ulcerate. Common signs include tenesmus, constipation, pain, anorexia, and weight loss. Secondary infection is likely and is often associated with pruritus.
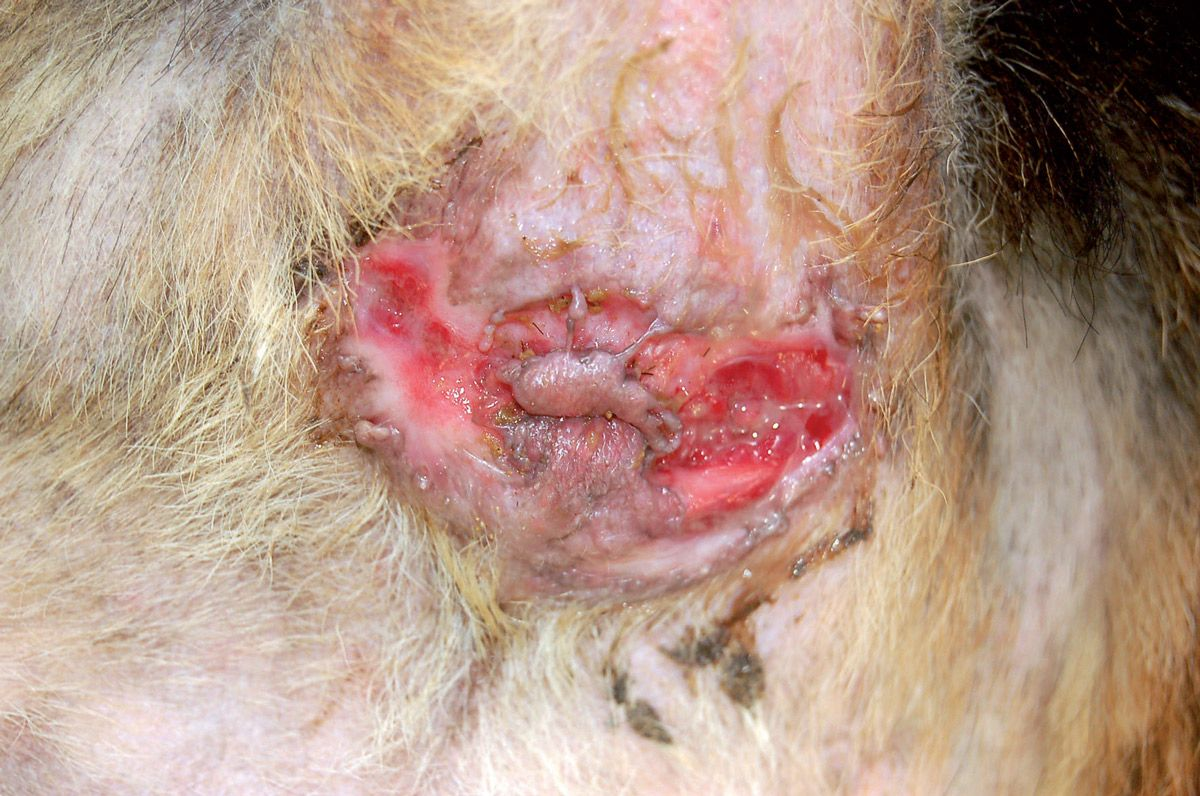
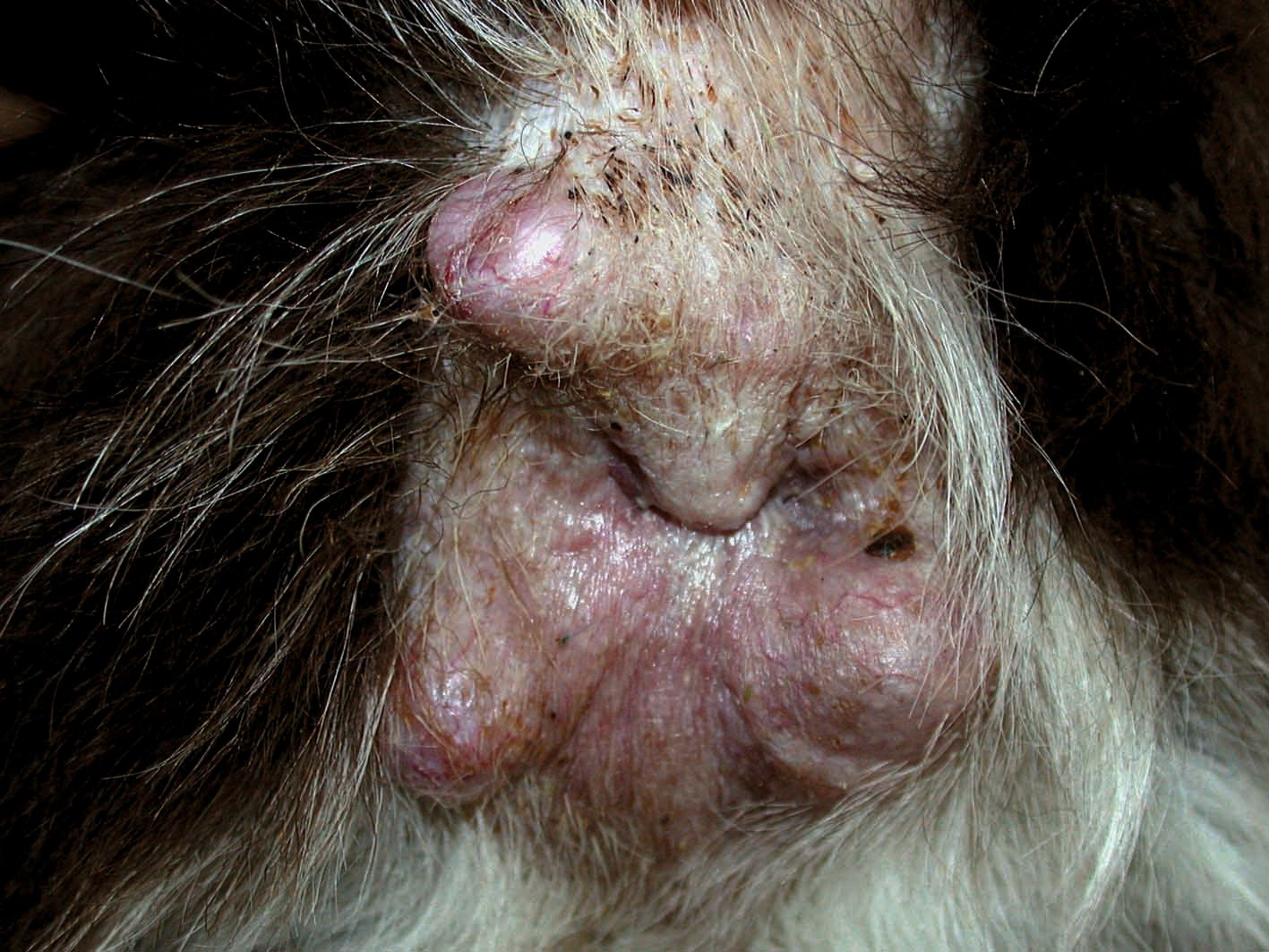
Other non-dermatological causes
Other less frequent conditions have been associated with perianal pruritus, including rectal diseases, gastrointestinal conditions (e.g., colitis) [15], psychological and metabolic factors [7] and drug reactions (including diarrhea related to medications).
Dermatological causes
Allergies
A recent study investigated the association between perianal pruritus and skin diseases in dogs without gastroenteric, anal/perianal or rectal diseases [1]. 92 out of 250 (37%) dogs presented to a dermatological specialist had perianal pruritus and it was found significantly more often in cases with atopic dermatitis (52% of affected dogs) and/or adverse food reactions (51% of affected dogs) than in all other skin diseases, in line with a previous study [16]. Flea bite hypersensitivity has also been associated with perianal pruritus, with a prevalence ranging from 9-67% [1],[17].
Other skin diseases
Although less common than allergies, other skin disorders such as sarcoptic mange, demodicosis, keratinization defects, sebaceous adenitis and contact dermatitis can be associated with perianal pruritus. Furthermore, immune-mediated diseases, such as pemphigus foliaceus and mucocutaneous lupus (Figure 4), and neoplasias, such as epitheliotropic lymphoma and mast cell tumor, can also affect the anal and perianal skin and occasionally cause itching.
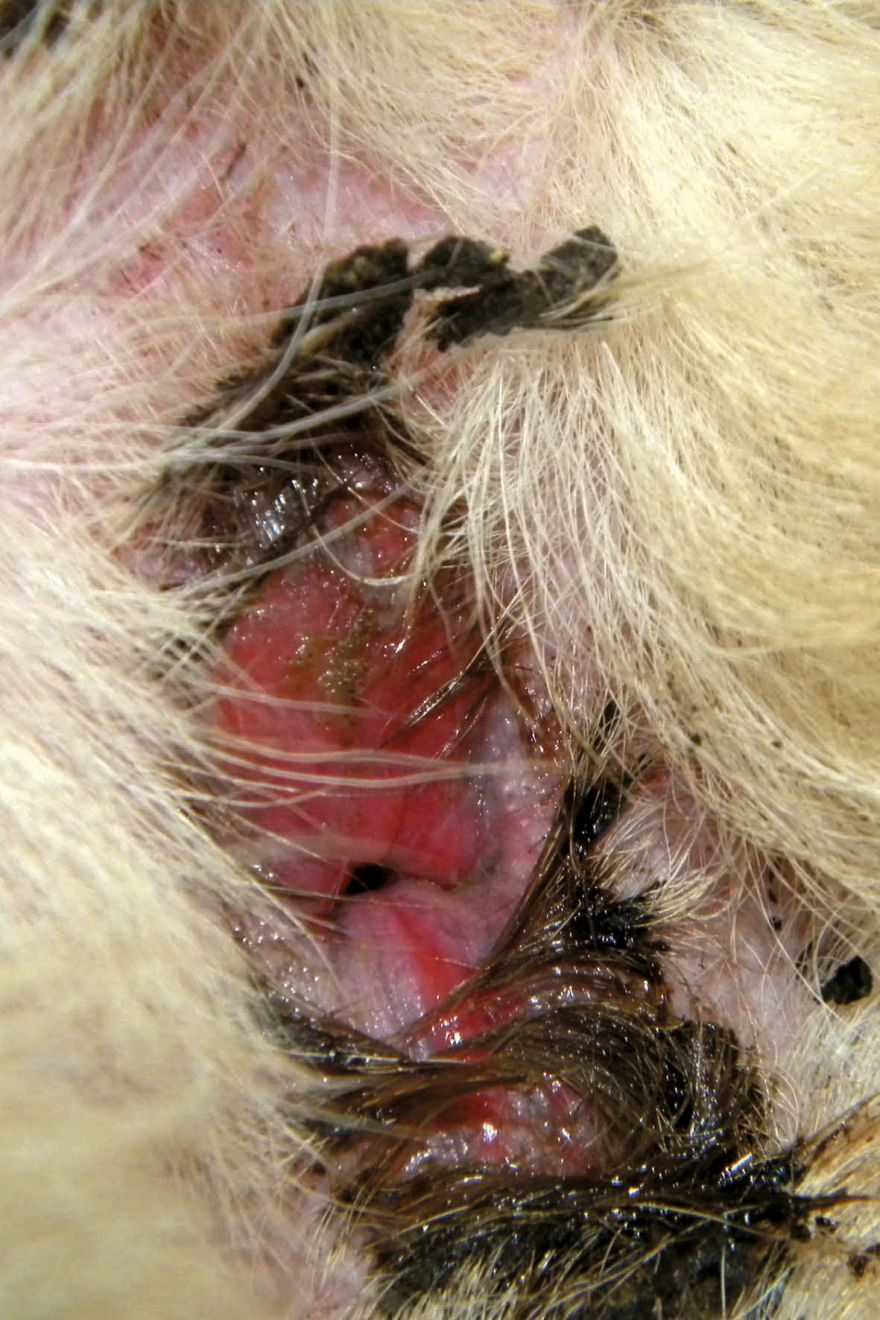
Diagnostic approach
To determine the correct diagnosis a methodical work- up should be initiated: it is important that all differentials are considered during the collection of the history and the clinical examination. The diagnosis is achieved by excluding other possible etiological factors.
Signalment and history
Breed, age and sex can give important clues for the diagnosis. Some diseases may have a breed predisposition, such as perianal furuncolosis in the German Shepherd dog or allergic dermatitis in the West Highland White Terrier and Labrador Retriever. Onset of clinical signs at an early age (< 1 year of age) is suggestive of parasitoses or food allergy. Anal sac carcinoma may be more often diagnosed in females, and hepatoid gland tumors are more frequent in intact male dogs.
It is important to collect information on the clinical presentation of the pruritus. Recurring pruritus in the warmer months may suggest seasonal atopy or flea bite hypersensitivity. If the itch improves after the anal sacs are squeezed, anal sac impaction is the more probable cause. If other areas of the body are itchy, such as paws, groin, axillae or ears, atopy or food allergy could be to blame, while if pruritus is localized mainly on the back and tail base fleas and/or flea bite hypersensitivity may be the most likely diagnosis. The clinician should also accurately assess the dog’s behavior: it has been speculated that licking or chewing at the anal region without scooting may be more indicative of an allergic disease than of ASD [1].
Establish if there are concomitant gastroenteric abnormalities. If the dog has a history of excessive bowel movements alone or with chronic flatulence, and if signs such as vomiting, diarrhea, constipation, tenesmus and/or dyschezia are present, then gastrointestinal problems such as colitis, intestinal parasites, adverse food reactions and intestinal bowel diseases (IBD) should be considered. To highlight concurrent food-related disorders such as adverse food reactions, colitis and IBD, the history should also consider the current diet and any previous modifications. In humans, contact dermatitis (from soap, toilet papers or creams) is a common cause of perianal pruritus. In dogs this is less frequent, but it is always worth asking if topical products, such as cleansing wipes, have been used. Previous administration of drugs, including anti-parasitic products, should be also investigated and the pharmacological history should be detailed.
Examination
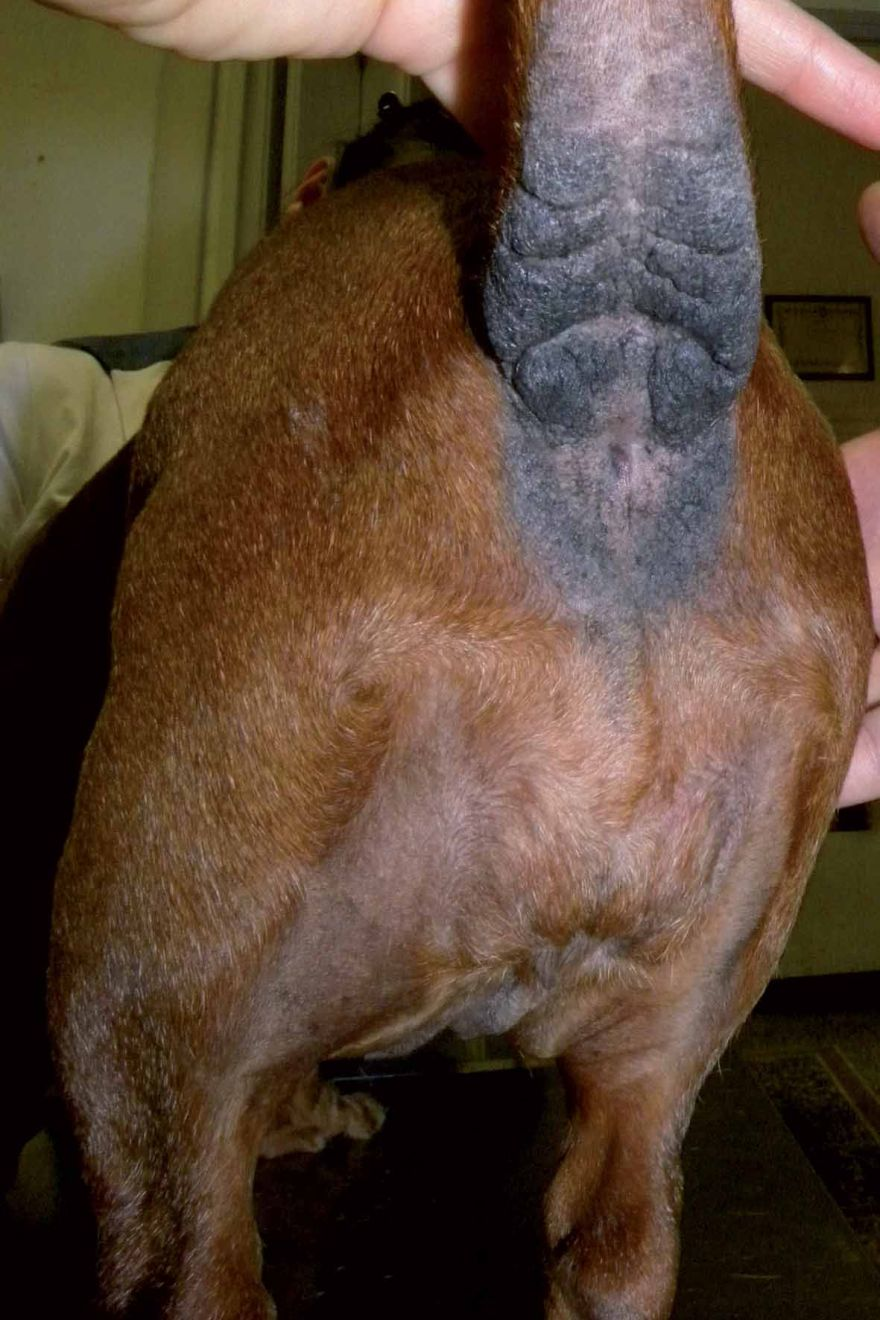
A general clinical examination, checking for systemic signs, should be followed by a full dermatological evaluation, looking for evidence of skin lesions and/or parasites in all areas of the body. Finally, the clinician should focus on the perianal area, looking for both primary and secondary lesions. Perianal erythema (Figure 5) and excoriation, as well as alopecia, hyperpigmentation and lichenification (Figure 6) are common sequelae of acute and chronic inflammation respectively. Presence of such lesions in the perianal region is strongly associated with perianal pruritus [1].
The anal orifice and the surrounding skin may be affected by fistulae (Figure 2), swelling (Figure 1) or nodules (Figure 3), as seen in perianal furuncolosis or neoplasia. Emerging proglottids, indicative of tapeworm infestation, may be present. A digital anorectal examination should follow, to assess the presence of indurations, nodules or purulent or hematogenous exudate. The anal sacs should then be gently squeezed to evaluate the presence, color and consistency of the secretion, and cytological evaluation of the contents should be undertaken. If the perineal area is highly inflamed and painful, it is advisable to apply a local anesthetic cream, or even sedate the patient, before doing any physical examination.

Ancillary testing
Cytology is useful for diagnosing infection or neoplasia. In the perianal skin the presence of Malassezia dermatitis or pyoderma can be best assessed by tape imprints, stained and examined under light microscopy. A small amount of anal sac secretion from each side should be placed on a glass slide, allowed to dry and then stained: the presence of neutrophils may indicate an anal sac infection or a pyoderma [5].
Cytology is indicated to investigate nodules and palpable lymph nodes; the presence of degenerate neutrophils together with phagocytosed bacteria suggests infection, e.g., an anal sac abscess, whilst neoplasia may be suspected when cytology shows a monomorphic population of non-inflammatory cells.
Biopsy is indicated when cytological examination is suggestive of neoplasia or immune-mediated disease, or when lesions do not respond to an apparently appropriate therapy. Fecal examination and broad-spectrum antiparasitics will be useful in the diagnosis of intestinal parasites. Blood sampling may be useful in some cases, e.g., hypercalcemia can be indicative of anal sac carcinoma.
Strict flea control can help identify a flea bite allergy, while an 8-week long elimination food trial, followed by provocative challenge, can help to diagnose a food allergy. Elimination food trials can be performed by feeding a home-cooked or limited antigen diet with ingredients novel to the dog, or by feeding a hydrolyzed diet. If all previous tests prove negative, then the dog most probably has atopic dermatitis. A symptomatic, non-sedating treatment for pruritus (e.g., oclacitinib) can be used to discriminate atopic dermatitis from behavioral disorders.
Therapy
Etiological treatment
For a successful, long-term cure, it is necessary to control and treat the underlying etiology responsible for the perianal pruritus. A detailed description of all therapeutic interventions for the various cited underlying etiologies is beyond the scope of this article, but it is appropriate to focus on the most typical or frequent causes of pruritus affecting the anal and perianal area.
Anal sac impaction is best treated by frequent manual expression of the sacs [7]; a finger is introduced into the anus and the sac is expressed with delicate pressure between the finger and thumb. This method permits the complete emptying of both sacs. Alteration of the diet, e.g., integration with prebiotics to improve the consistency of the stools may promote natural emptying.
Anal sac infection is treated by emptying and flushing the sacs; this can be painful and may require sedation. The sacs are catheterized using a round-ended catheter (e.g., a cat urinary catheter) and flushed with isotonic saline [7]; a suitable antibiotic solution (based on culture results if available) is then introduced. Various antibiotic combinations can be employed but chloramphenicol has been shown to have a broad spectrum of activity against the common pathogens. Corticosteroids may also be infused if appropriate. If Malassezia is present then the use of nystatin or an imidazole derivative (miconazole, clotrimazole) is indicated.
When an anal sac abscess is present, there is a risk of rupture with drainage to the perianal skin or into the rectum. In this case systemic antibiotics are indicated, preferably based on sensitivity testing, although topical treatment (drainage and lavage with 0.5% chlorhexidine or 10% povidone iodine and instillation of an antibiotic solution) can also be useful. Surgical removal of the sacs is advised in cases of frequently recurring anal sacculitis or abscesses [7].
Perianal furunculosis is best treated with oral antibiotics, cyclosporine (5-10 mg/kg q12-48H [18]) and/or topical 0.1% tacrolimus [19] given until 4-8 weeks after resolution. Administration of ketoconazole (2-10 mg/kg q12-24H) improves the efficacy of cyclosporine and can reduce its dose (and possibly cost) by up to 50% [20]. Relapses and incomplete resolutions are frequently seen, and alternate day permanent maintenance therapy may be necessary in some cases [21].
Flea infestation and flea bite hypersensitivity require a strict flea control program. Food allergy is best controlled by avoidance of the specific offending food, preferably by means of a complete well-balanced commercial limited-antigen or hydrolyzed protein diet [15]. Causative factors responsible for contact dermatitis or allergy may be identified by patch testing and further avoided if possible. Atopic dogs can be controlled with allergen-specific immunotherapy [21] or symptomatic treatments against pruritus (see below).
Symptomatic treatment
In many cases, in order to decrease pruritus and improve the quality of life for both dogs and owners, symptomatic treatment of pruritus may be necessary. Topical antipruritic therapy is usually based on corticosteroid cream or solution. Several studies have confirmed the efficacy of a commercial hydrocortisone spray [22] which is easy to administer and is indicated for managing both acute and chronic pruritus [22]. It is well tolerated and safe: skin thinning, which is a side effect often associated with prolonged topical corticosteroids, has not been reported with daily application of this product [23].
Systemic antipruritic drugs, such as cyclosporine (5 mg/ kg q24H given for one month, then tapered to every other day [24]), or oclacitinib (0.4-0.6 mg/kg q12H for two weeks, then tapered to q24H [25]), can be the best options for long-term management in many cases.
Conclusion
Perianal pruritus is a complaint commonly reported by dog owners and a distressing condition for the pet. Although there are many different etiologies, it is most often linked to anal sac disease or allergic dermatitis; however, the clinician should always perform a systematic diagnostic approach to identify and, when possible, remove the cause.
References
- Maina E, Galzerano M, Noli C. Perianal pruritus in dogs with skin disease. Vet. Dermatol. 2014;25:204-209.
- Williams LE, Gliatto JM, Dodge RK, et al. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985-1995). J. Am. Vet. Med. Assoc. 2003;223:825-831.
- Esplin DG, Wilson SR, Hullinger GA. Squamous cell carcinoma of the anal sac in five dogs. Vet. Pathol. 2003;40:332-334.
- Hedlund CS, Fossum TW. Anal sac infection and impaction. In: Fossum TW, ed. Small Animal Surgery, 3rd ed. St Louis, MO: Mosby, 2007;498,511-515.
- Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, ed. Tumors in domestic animals. 4th ed. Iowa: Ames; 2002;44-117.
- Walder EJ, Gross TL: Epithelial tumors. In: Gross TL, Ihrke PJ, Walder EJ, eds. Veterinary Dermatopathology: a macroscopic and microscopic evaluation of canine and feline skin disease. Boston: Mosby 1992;329-520.
- Guilford WG. Adverse food reactions. In: Guilford WG, Center SA, Strombeck DR, et al, eds. Strombecks Small Animal Gastroenterology. 3rd ed. Philadelphia, PA: WB Saunders Co, 1996;436-450.
- Favrot C, Steffan J, Seewald W, et al. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet. Dermatol. 2010;21:23-31.
- Bruet V, Bourdeau PJ, Roussel A, et al. Characterization of pruritus in canine atopic dermatitis, flea bite hypersensitivity and flea infestation and its role in diagnosis. Vet. Dermatol. 2012;23:487-493.
- Griffiths LG, Sullivan M, Borland WW. Cyclosporine as the sole treatment for anal furunculosis: preliminary results. J. Small Anim. Pract. 1999;40:569-572.
- Stanley BJ, Hauptman JG. Long-term prospective evaluation of topically applied 0.1% tacrolimus ointment for treatment of perianal sinuses in dogs. J. Am Vet. Med. Assoc. 2009;235:397-404.
- James DJ, Griffin CE, Polissar NL, et al. Comparison of anal sac cytological findings and behaviour in clinically normal dogs and those affected with anal sac disease. Vet. Dermatol. 2011;22:80-87.
- Patricelli AJ, Hardie RJ, McAnulty JE. Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. J. Am. Vet. Med. Assoc. 2002;220:1009-1016.
- Hardie RJ, Gregory SP, Tomlin J, et al. Cyclosporine treatment of anal furunculosis in 26 dogs. J. Small Anim. Pract. 2005;46:3-9.
- Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet. Dermatol. 2010;21:233-248.
- Nuttall TJ, McEwan NA, Bensignor E, et al. Comparable efficacy of a topical 0.0584% hydrocortisone aceponate spray and oral cyclosporine in treating canine atopic dermatitis. Vet. Dermatol. 2012;23:4-10.
- Olivry T, Bizikova P. A systematic review of randomized controlled trials for prevention or treatment of atopic dermatitis in dogs: 2008-2011 update. Vet. Dermatol. 2013;24:97-117.
- Cosgrove SB, Wren JA, Cleaver DM. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet. Dermatol. 2013;24:479-e114.
- Little SE, Johnson EM, Lewis D, et al. Prevalence of intestinal parasites in pet dogs in the United States. Vet. Parasitol. 2009;166:144-152.
- Georgi JR, Georgi ME. Helminths. In: Parasitology for Veterinarians. 5th ed. Philadelphia: WB Saunders Co, 1990;137.
- Pappalardo E, Martino PA, Noli C. Macroscopic, cytological and bacteriological evaluation of anal sac content in normal dogs and in dogs with selected dermatological diseases. Vet. Dermatol. 2002;13:315-322.
- Hill PB, Lo A, Eden CA, et al. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. Vet. Rec. 2006;158:533-539.
- Muse R. Diseases of the anal sac. In: Bonagura JD, Twedt DC, eds. Kirks Current Veterinary Therapy XIV. St Louis, MO: Saunders, 2008;465-468.
- Halnan CR. The diagnosis of anal sacculitis in the dog. J. Small Anim. Pract. 1976;17:527-535.
- Vercelli A. Perianal diseases in dogs. In Proceedings: Eur. Soc. Vet. Dermatol. Eur. Col. Vet. Dermatol. 1997;14:51-55.
Elisa Maina
DVM, PhD, Dip. ECVD
Dr. Maina is a Diplomate of the European College of Veterinary Dermatology and also holds a PhD for research in immunology. She graduated from Milan’s Faculty of Veterinary Medicine in 2008 and went on to study dermatology with the European School of Advanced Veterinary Studies before completing a dermatology externship at the University of Florida and a residency in Italy. She currently works in a Swiss referral clinic, but also finds time to author papers for various international dermatology journals, and she serves as Chair of the ECVD Credentials Committee.
Chiara Noli
DVM, Dip. ECVD, Servizi Dermatologici Veterinari, Peveragno, Italy
Dr. Noli graduated in veterinary medicine from the University of Milan in 1990. After a residency in Utrecht, she obtained her Diploma in Veterinary Dermatology in 1996, and she currently works as a referral dermatologist in Italy. A board member and past-President of the European Society of Veterinary Dermatology, she has lectured extensively on the subject, and has authored many articles in national and international journals, as well as contributing to various book chapters; she is co-editor of the recently published textbook “Veterinary Allergy”.
Other articles in this issue
Share on social media