Systemic implications of periodontal disease
Written by Alessandro De Simoi
Periodontal disease is the most common infectious disease found in small animals with a prevalence that approaches 80%; prevalence increases with age and diminishes as body size increases – it is much more common in smaller animals compared to those of medium and large size.
Article
Key Points
Periodontal disease is the most common infectious disease found in small animals.
It has been suggested that periodontal disease can be a major factor in various systemic illnesses including cardiovascular problems, reproductive disorders, liver disease and diabetes.
Various hypotheses have been suggested as to how periodontitis may influence systemic disease but as yet there is no definitive proof of a link.
Periodontal disease can be prevented by meticulous removal of bacterial plaque with tooth brushing and oral hygiene.
Introduction
Periodontal disease is the most common infectious disease found in small animals with a prevalence that approaches 80% [1]; prevalence increases with age and diminishes as body size increases – it is much more common in smaller animals compared to those of medium and large size [1]. The periodontium comprises the gingiva, cementum, dental alveolar ligament and alveolar bone, which together contribute to the support of the tooth. Periodontal disease is caused by bacterial plaque, and can be subdivided into two parts: gingivitis and periodontitis. Gingivitis is a reversible inflammation of the gums, because once the cause (bacterial plaque) has been removed, the inflammation recedes. Periodontitis, on the other hand, is an irreversible inflammatory condition of non-gingival tissue (the alveodental ligament, cementum and alveolar bone) and is assessed by measuring the loss of attachment of the tooth. Periodontitis may be either inactive (quiescent), where there is no evidence of gingival inflammation (and if there has been loss of tooth attachment this may have occurred some time previously), or active, where there is ongoing destruction of tissue (Figure 1). Although it is recognized that periodontitis is an infectious disease, and more than 700 bacterial species have been identified as being capable of colonizing the biofilm of the subgingival sulcus, Koch’s postulates* do not apply [2].
* 1. The microorganism must be found in abundance in an animal suffering from the disease, but should not be found in healthy animals. 2. The microorganism must be isolated from a diseased animal and grown in culture. 3. The cultured microorganism should then cause disease when introduced into a healthy animal. 4. The microorganism must be re-isolated from this experimentally infected animal and identified as being identical to the original causative agent.
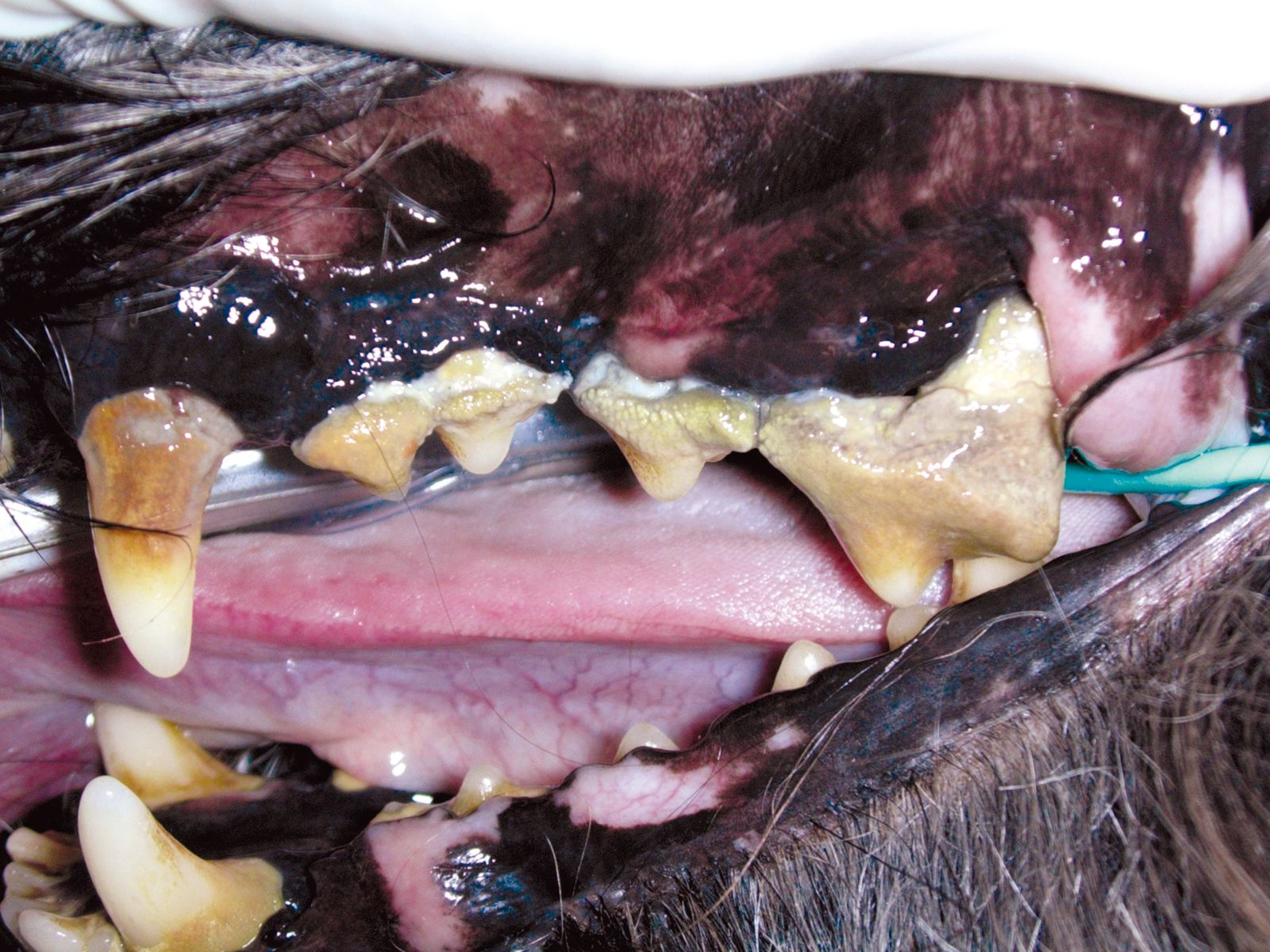
Gingivitis, even if untreated, does not always result in periodontitis; the development of periodontal disease is in fact determined by an imbalance between the bacterial population and the host immune system. Immune capability, stress, age, nutritional and metabolic status, breed and endocrine disease are all factors that may aid or prevent the progression of periodontal disease. If the disease progresses, the bony destruction and apical migration of the supporting connective tissue will cause loosening and eventual loss of one or more teeth.
Periodontal disease is a focal infection. This concept, introduced more than a century ago, describes a localized, chronic disease, which represents a source of microorganisms, toxins, and products of bacterial and tissue degradation, capable of reaching distant organs and tissues [3]. The surface area involved in periodontitis has been measured in affected toy breeds and found to range between 3.18-29.8 cm2 [4] and so the area of diseased tissue can represent a considerable proportion of the dog’s total body surface area.
During the development of periodontitis the bacteria present in periodontal pockets can reach the bloodstream, causing bacteremia, and, although in healthy individuals these are intercepted by the reticuloendothelial system [5], the continuous prolonged exposure to bacteremia may be associated with systemic disease associated with distant organs and systems [6] [7]. The systemic implications of periodontal disease are not, however, limited to the bacterial load. Inflammatory chemical mediators, bacterial endotoxins and toxins from tissue degradation can also be involved, either by direct harmful effects or because they cause immune reactions in organs distant to the oral cavity.
Cardiovascular implications
The same is true in veterinary medicine, where studies have also demonstrated a positive correlation between the presence of periodontal disease and histopathologic changes affecting the heart and other internal organs [4] [5] [6] [7][11] [12] [13] Nevertheless, international scientific opinion is not unanimous on the importance of oral infections in the genesis of systemic disease; this is because there is currently no conclusive evidence of a direct link between periodontal disease and other diseases [14].
Reproductive disorders
It has been shown that pregnant women with periodontitis are up to 7.5 times more likely to give birth prematurely, and for their babies to have low birthweight. This finding correlates with the increase in pro-inflammatory cytokines triggered by circulating bacterial lipoproteins. In some cases periodontopathic bacteria were detected directly within the amniotic fluid [15].
Diabetes mellitus
Liver disease
Etiopathogenic hypotheses
Given that it is difficult to clearly identify the mechanisms that link oral and systemic disease, different hypotheses have been proposed to explain this relationship, namely direct infection, systemic inflammation with endothelial damage, and molecular mimicry between bacterial antigens and autoantigens.
Direct infection hypothesis
Systemic inflammation hypothesis
According to this hypothesis periodontitis causes an increase in circulating cytokines which can directly damage the endothelium of blood vessels, leading to the formation of lesions that affect the heart and other internal organs. It has been demonstrated that pro-inflammatory cytokines such as TNF and IL6 can cause anabolic mutations in myocytes through the activation of intracellular signals leading to myocardial hypertrophy [20]. In several studies high levels of CRP have been observed in cases of chronic periodontitis [21] whilst one recent study [22] demonstrated that people who underwent intensive periodontal therapy (scaling and root planing) had significantly reduced elasticity of their brachial artery 24 hours post-therapy when compared to a control group. This was related to the increase in CRP and IL6 during periodontal therapy. However 60 and 180 days after the dental work, the vascular elasticity was significantly greater in the group subjected to periodontal therapy compared to the control group; this increase was attributed to the beneficial effects of the periodontal treatment.
Molecular cross-reactivity hypothesis
Diagnosis of periodontal disease
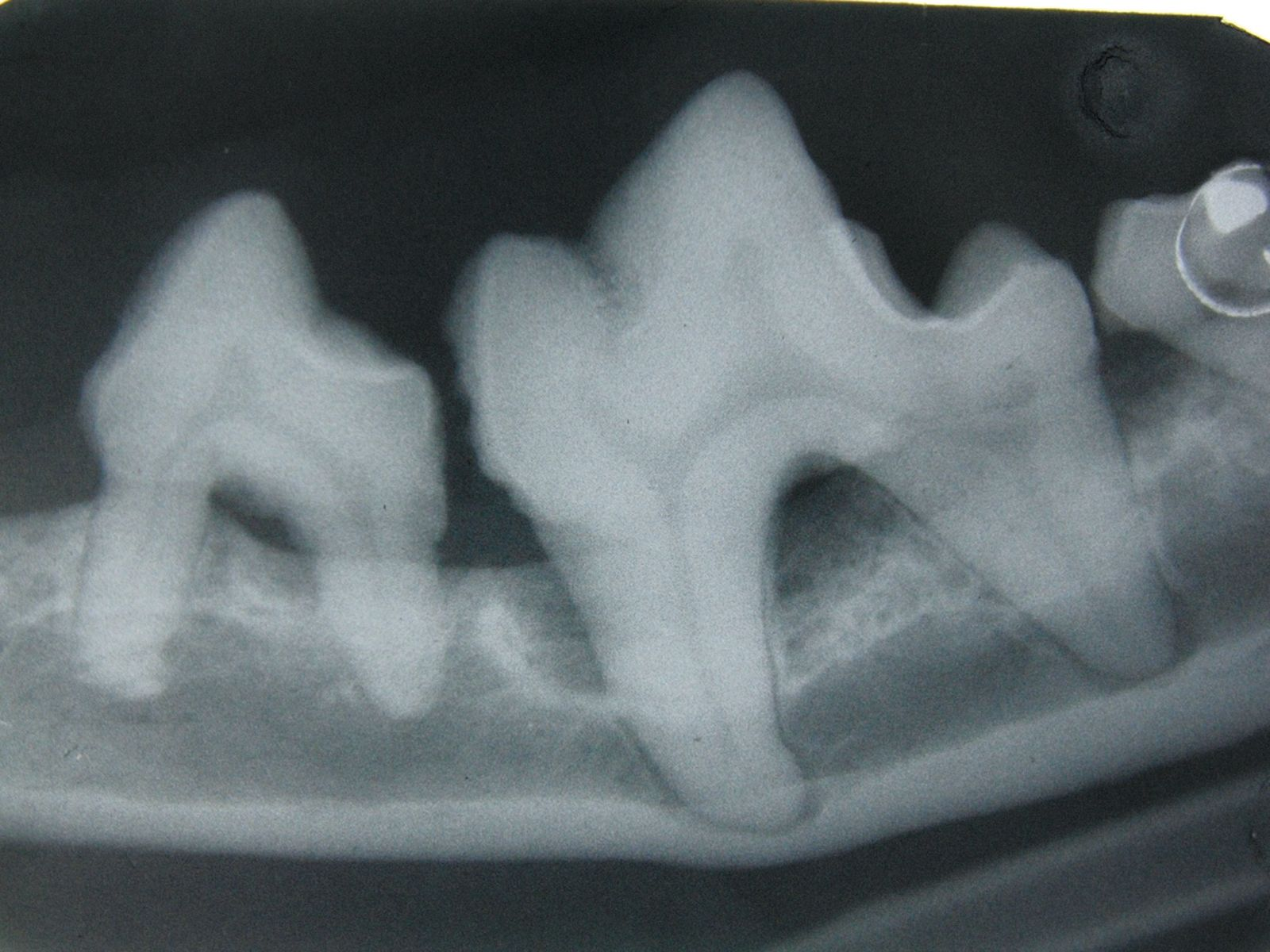
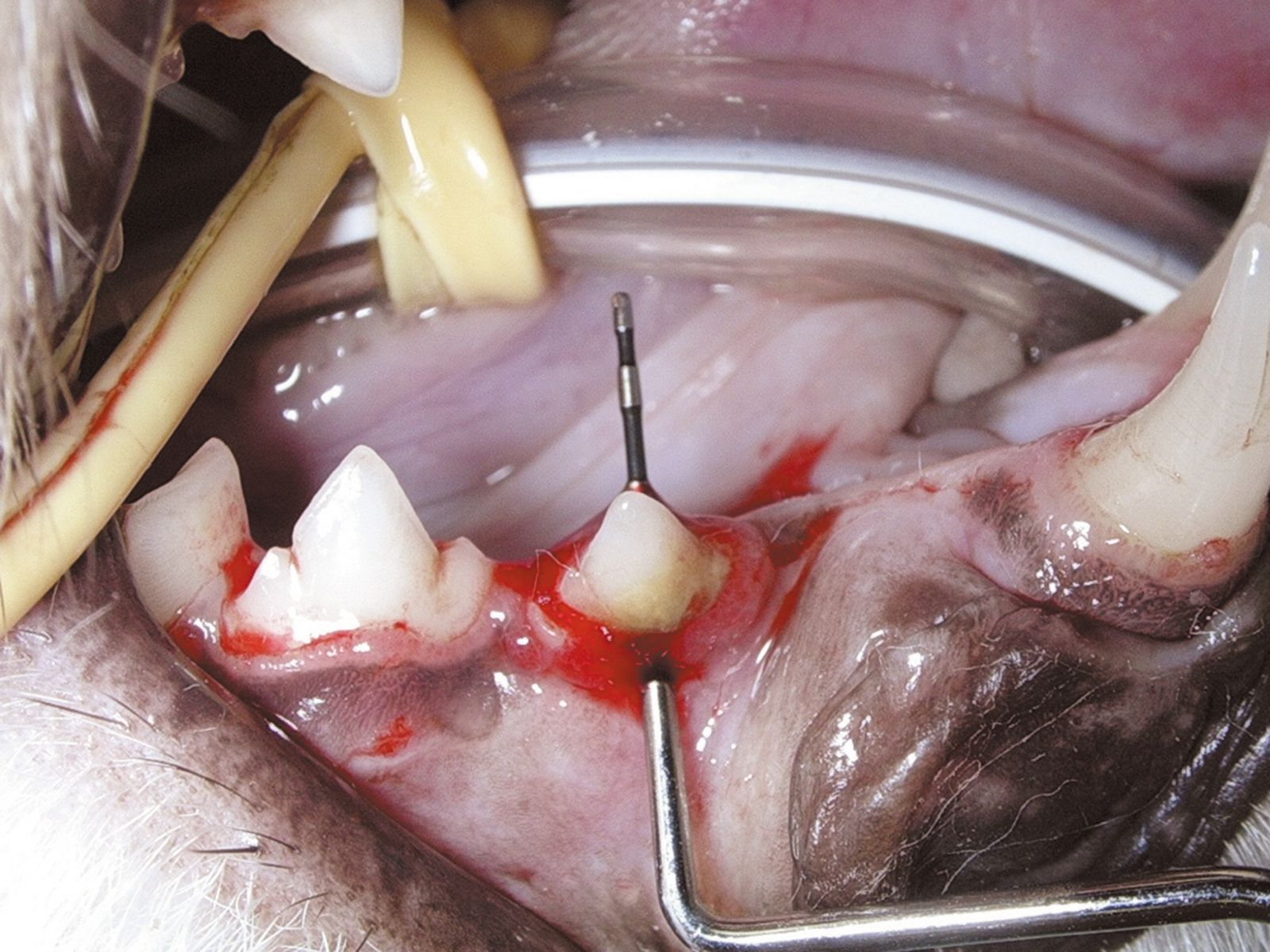
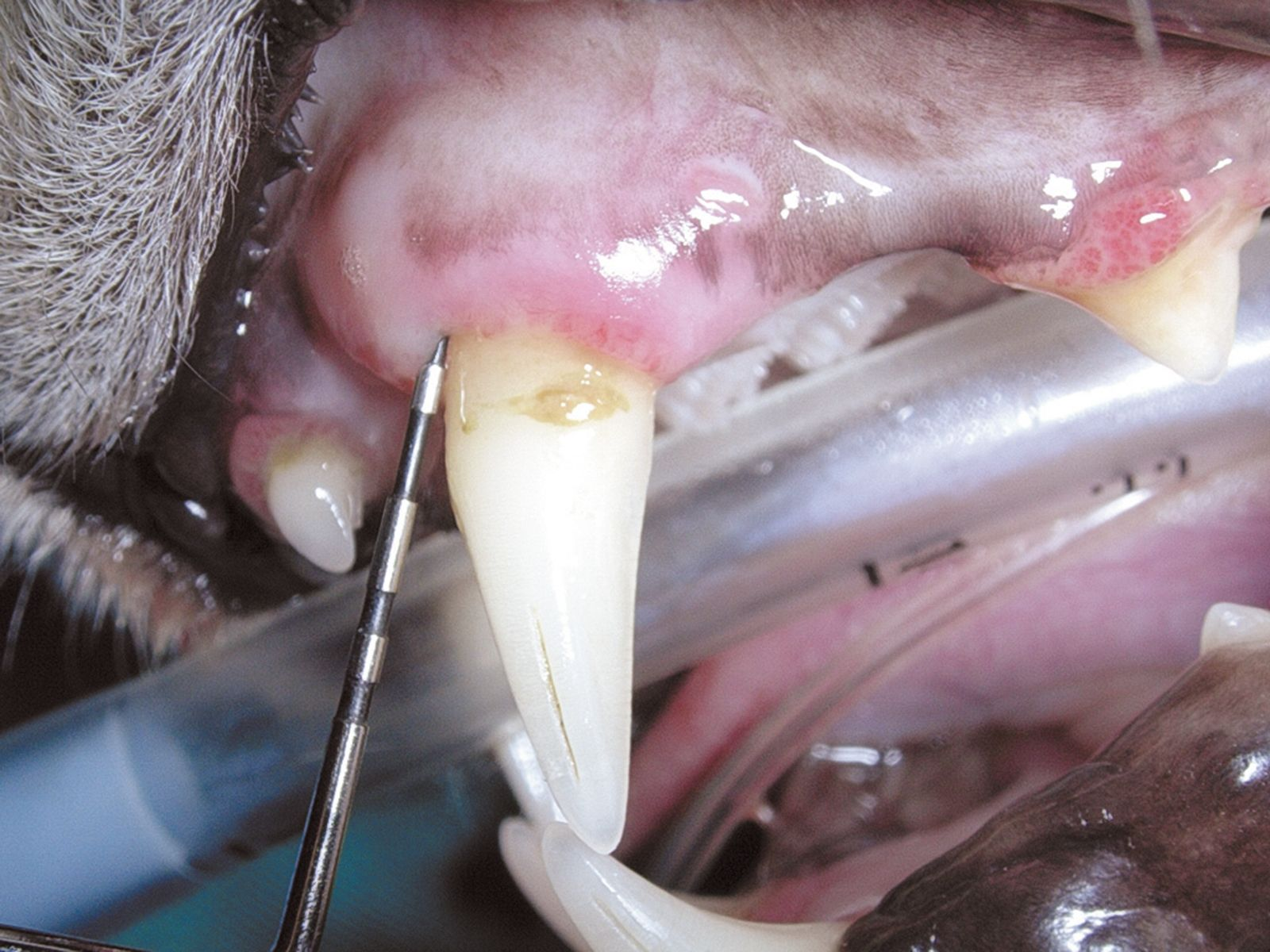
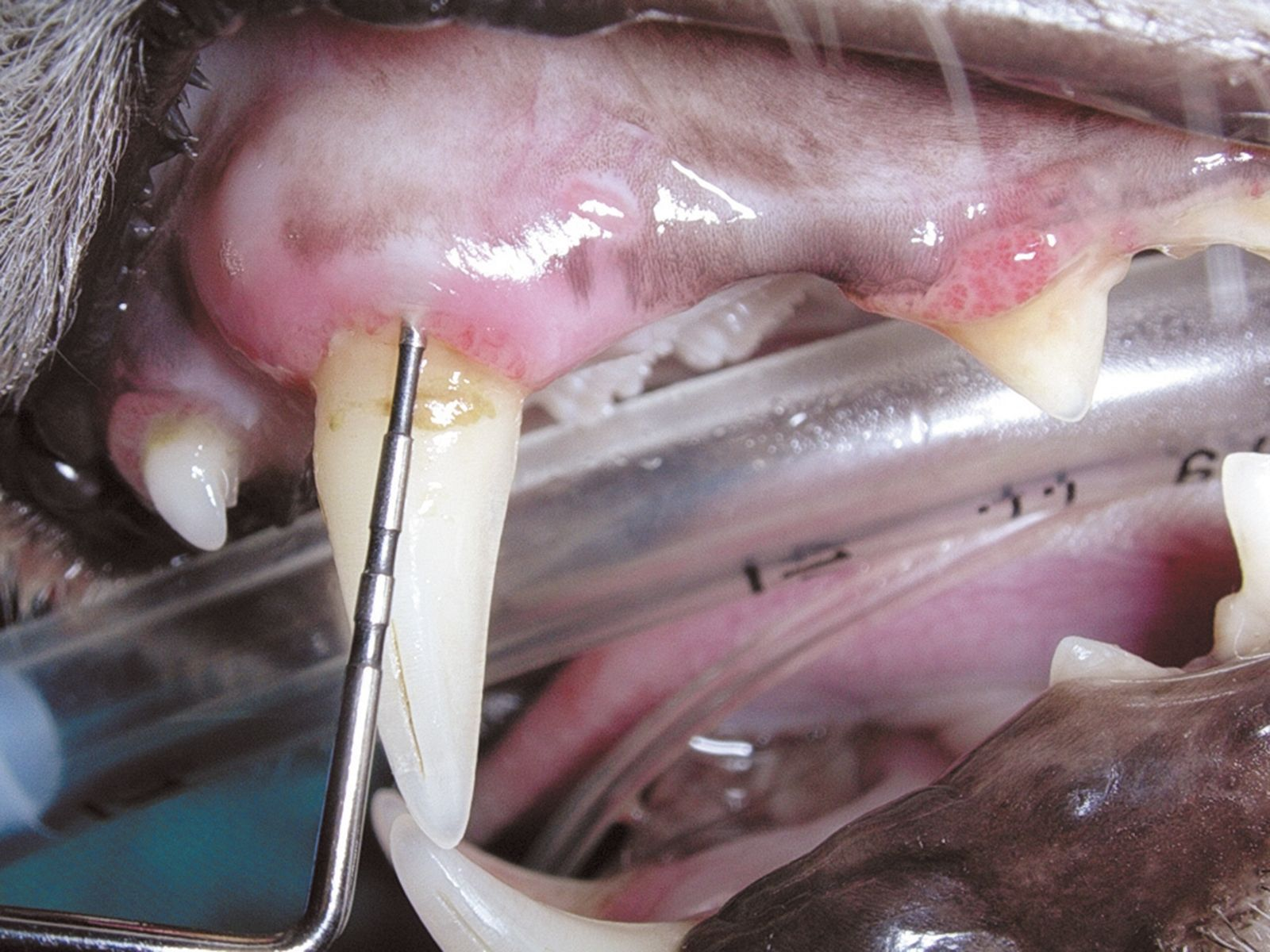
Generally, periodontal disease starts with few or no clinical signs and the main reason for the owner to request an evaluation of the oral cavity is because their pet has developed halitosis. Accurate diagnosis cannot be based solely on visual inspection of the oral cavity; general anesthesia followed by a periodontal examination involving the use of a probe (Figure 2) and intraoral radiography is essential. Various periodontal probes are available but all are designed to allow pocket depth measurement and assessment of gingival hyperplasia or recession. With the probe one can also evaluate the degree of tooth mobility and the presence of furcation lesions in double or triple rooted teeth (Figure 3). The probe should be gently introduced into the gingival sulcus (Figures 4-5), ideally evaluating 4-6 points around the circumference of each tooth; apparently healthy buccal teeth may have deep pockets either palatally or lingually. All observations must be recorded on a dental chart in order to provide an overall assessment.

Prevention and treatment of periodontal disease
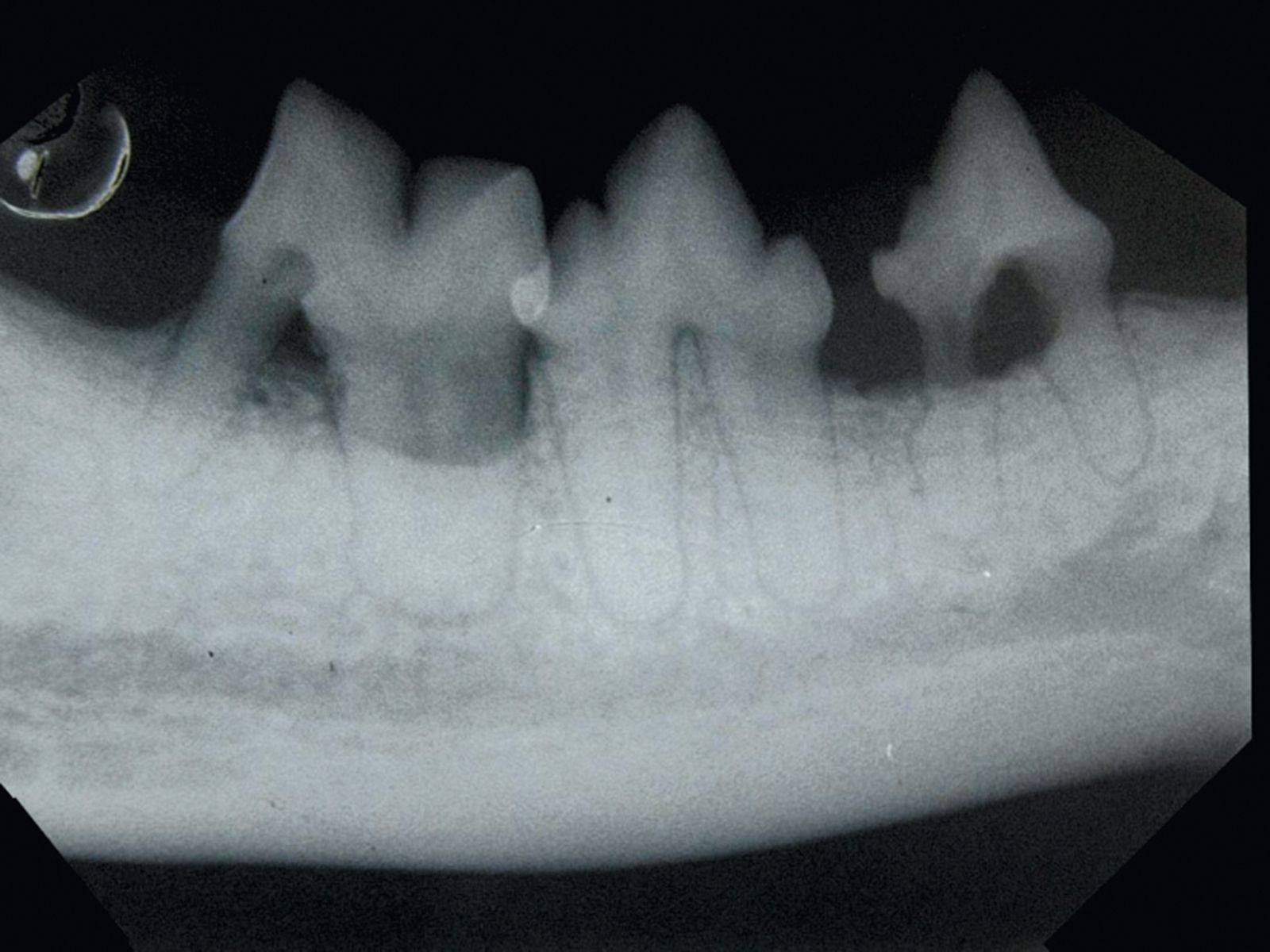
Periodontal disease can be prevented by meticulous removal of bacterial plaque with tooth brushing and oral hygiene. Some commercial diets may help in reducing coronal plaque but the crucial factor is the removal of subgingival plaque. The aim is not to sterilize the oral cavity but to prevent the bacterial biofilm changing from a mixed commensal population dominated by aerobic bacteria to a population that is predominantly anerobic. Treatment of periodontal disease should be performed with the animal anesthetized and the trachea intubated; once the arcades have been accurately charted and intra-oral radiographs have been analyzed (Figures 6-7) supra- and subgingival scaling is performed, followed by more complex procedures such as tooth extraction or periodontal surgery as indicated.
Antibiotic usage
Severe and extensive periodontal disease in an otherwise healthy subject should not be treated with antibiotics long term; the correct treatment is to remove the cause (plaque, tartar, irreparably compromised teeth) by scaling the teeth and performing tooth extraction as necessary. Antibiotics should be used for two main purposes: to treat local infection and to prevent bacteremia.Treatment of local infection
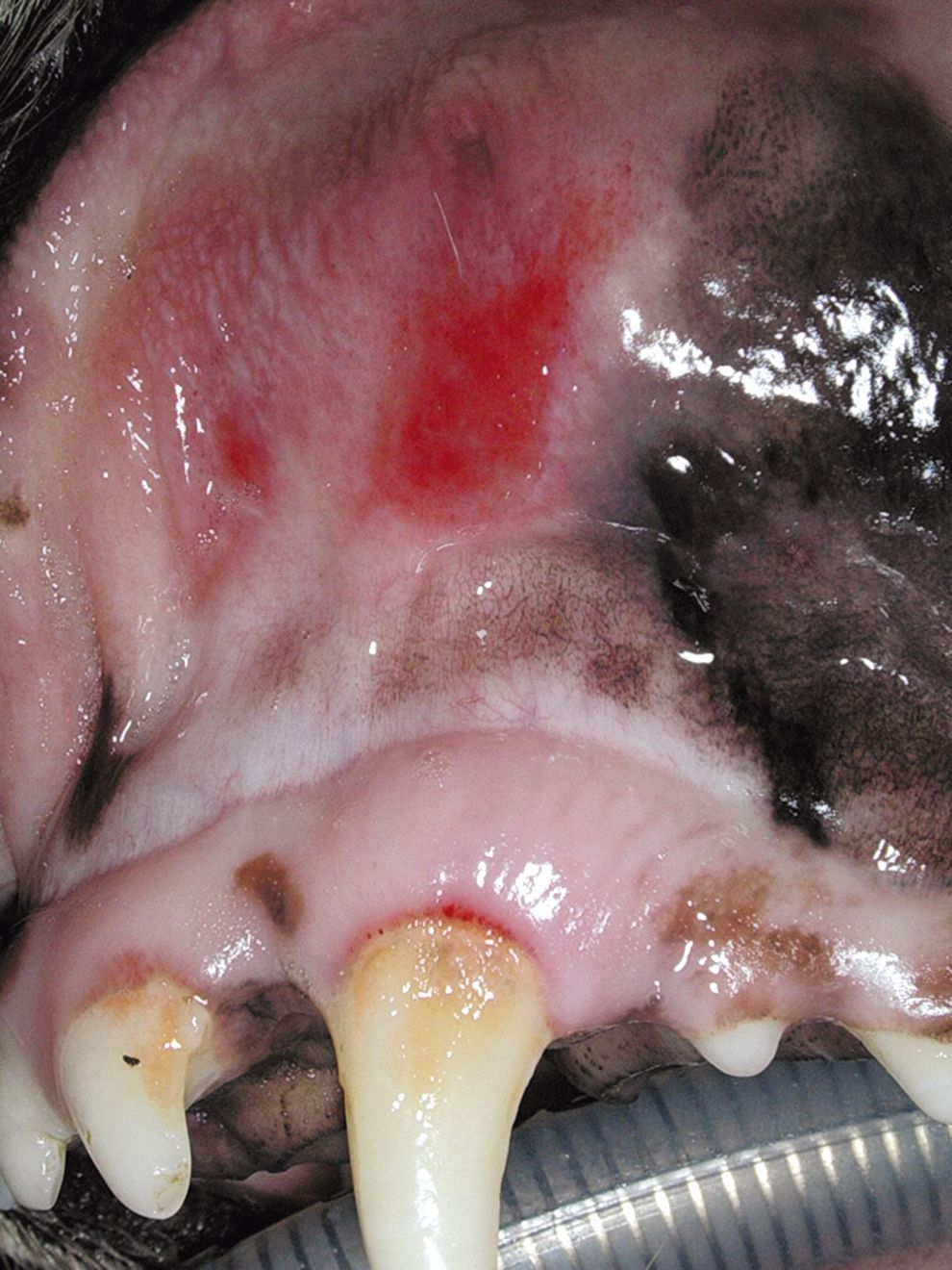
If periodontal disease has caused osteomyelitis of the maxilla or mandible, antibiotic therapy is recommended, starting a few days before surgery and continuing for several weeks afterwards. The use of antibiotics a few days before surgery is also indicated where ulcerative gingival lesions have developed (even if there is only a small accumulation of plaque), in cases of chronic ulcerative paradental stomatitis in dogs (Figures 8a and b), and in cats with stomatitis.
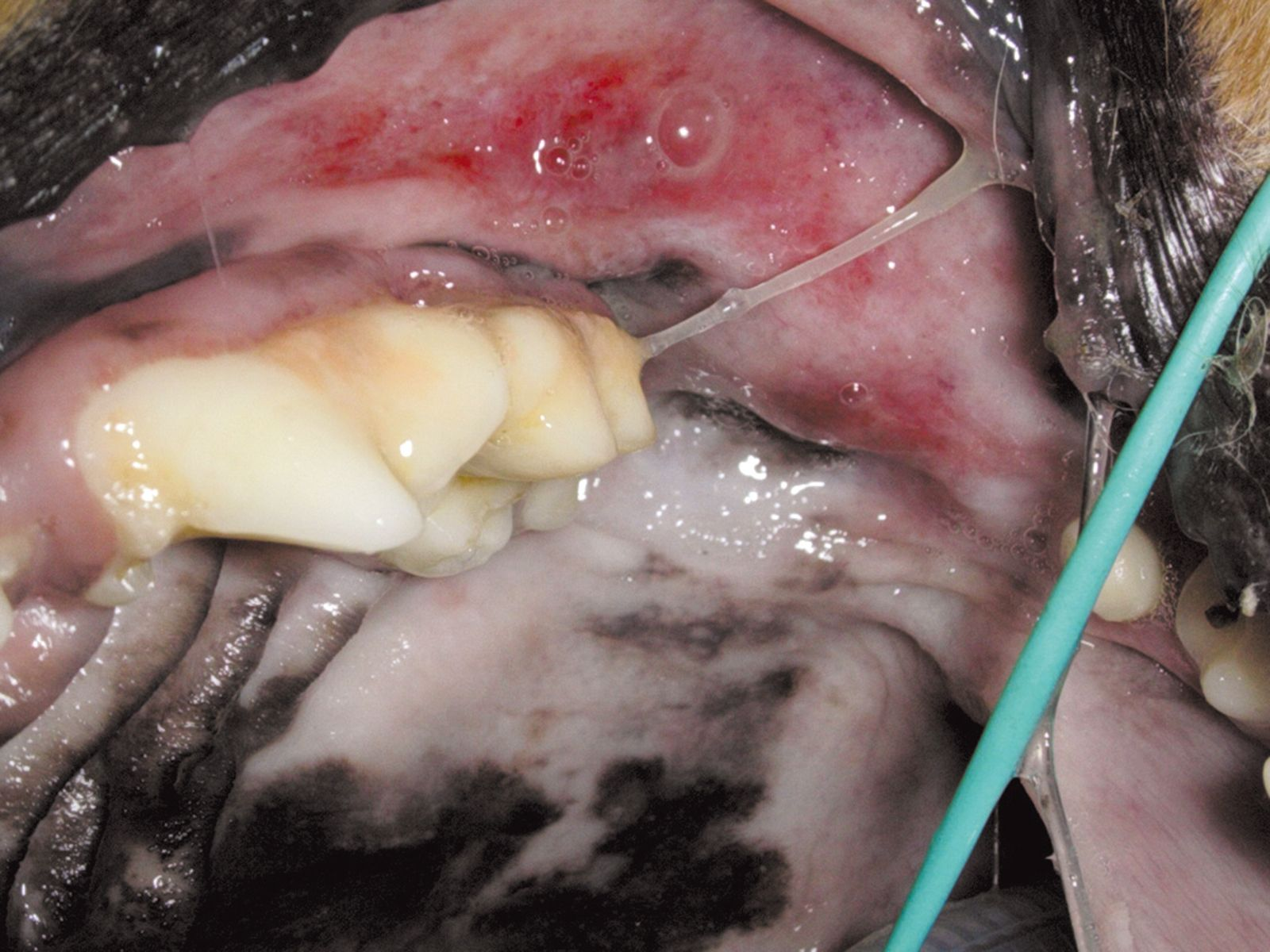
Prophylaxis of bacteremia
Bacteremia is common in patients with active gingivitis and periodontitis, from normal daily activities such as grooming and chewing of food. In healthy subjects this is quickly countered by the activity of the reticuloendothelial system. In the case of periodontitis patients with serious systemic disease such as heart problems, subjects with joint or eye replacements, splenectomized subjects or with hyperadrenocorticism, and when cellular metabolism is depressed by systemic disease, the risk that tissues distant to the mouth may be affected justifies the use of perioperative antibiotics. In these cases the choice is a broad-spectrum bactericidal antibiotic that can be administered intravenously at induction of anesthesia and repeated if the operation lasts more than two hours. It is also possible to administer a single dose orally on the morning of the surgery.Conclusion
At present there is no unequivocal evidence of a direct relationship between periodontal disease and systemic effects, even though there are various hypotheses that explain the relationship between the two conditions. However, much evidence suggests that periodontal disease may promote and maintain inflammation in organs distant to the mouth, and that even in the early stages of periodontitis the body can react with the synthesis of acute phase proteins, thereby demonstrating the stimulation of a systemic disease induced by inflammation within the oral cavity.
The health of the periodontium is not only important for the maintenance of the teeth. Periodontal disease can have a significant impact on overall health and may be responsible for morbidity and mortality, especially in certain susceptible dog breeds. Preventive measures such as oral hygiene, the use of chew toys, and the feeding of products specifically designed to reduce the accumulation of bacterial plaque and calculus should be considered when managing periodontitis. Dental health food products typically consist of a kibble with a conformation and structure that deliver a mechanical abrasive action, but some products also contain sodium polyphosphate which will chelate the salivary calcium, slowing the mineralization of plaque and consequently the formation of tartar. These foods may therefore be recommended as an aid in a general plan to reduce the risk of periodontal disease.
References
- Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999;214:1336-1341.
- Khader YS, Albashaireh ZSM, Alomari MA. Periodontal disease and the risk of coronary heart and cerebrovascular disease: a meta-analysis. J Periodontol 2004;75:1046-1153.
- Glickman LT, Glickman NW, Moore GE, et al. Association between chronic azotemic kidney disease and the severity of periodontal disease in dogs. Prev Vet Med. 2011 May 1;99(2-4):193-200. Epub 2011 Feb 23.
- Peddle GD, Drobatz KJ, Harvey CE, et al. Association of periodontal disease, oral procedures, and other clinical findings with bacterialendocarditis in dogs. J Am Vet Med Assoc. 2009 Jan 1;234(1):100-7.
- Yu G, Yu Y, Li YN, et al. Effect of periodontitis on susceptibility to atrial fibrillation in an animal model. J Electrocardiol. 2010 Jul-Aug;43(4):359-66. Epub 2009 Dec 29.
- O’Reilly PG, Claffey NM. A history of oral sepsis as a cause of disease. Periodontol 2000. 2000;23:13-18.
- Baskaradoss JK, Geevarghese A, Al Dosari AA. Causes of adverse pregnancy outcomes and the role of maternal periodontal status - a review of the literature. Open Dent J 2012;6:79-84. Epub 2012 May 9.
- van Nice E. Management of multiple dental infections in a dog with diabetes mellitus. J Vet Dent 2006;23(1):18-25.
- Yoneda M, Naka S, Nakano K, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol 2012;12:16.
- Ford PJ, GemmelE, Hamlet SM et al. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in arterosclerosis. Oral Microbiol Immunol 2005;20:296-302.
- Deshpande RG, KhanMB, Genco CA. Invasion of aortic and heart endothelial cells by Porphiromonas gingivalis. Infect Immun 1998;66:5337-5343.
- Haffajee AD, Socransky SS. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontol 2000,2006; 42:7-12.
- Franek E, Blach A, Witula A, et al. Association between chronic periodontal disease and left ventricular hypertrophy in kidney transplant recipients. Transplantation 2005;80:3-5.
- Amar S, Gokce N, Morgan S, et al. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol 2003;23:1245-1249.
- Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007;356:911-920.
- Polla BS. A role for heat shock proteins in inflammation? Immunol Today 1988;9:134-137.
- Wick G, Perschinka H, Xu Q. Autoimmunity and atherosclerosis. Am Heart J 1999;138:444-449.
- Ando T, Kato T, Ishihara K,et al. Heat shock proteins in the human periodontal disease process. Microbiol Immunol 1995;39:321-327.
- Vieira CL, Caramels B. The history of dentistry and medicine relationship: could the mouth finally return to the body? Oral Dis 2009;15(8):538-46.
- Pavlica Z, Petelin M, Juntes P, et al. Periodontal disease burden and pathological changes in organs of dogs. J Vet Dent 2008;25(2):97-105.
- Silver JG, Martin L, McBride BC. Recovery and clearance of oral micro-organism following experimental bacteremia in dogs. Arch Oral Biol 1975;20:675-9.
- Glickman LT, Glickman NW, Moore GE, et al. Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs. J Am Vet Med Assoc 2009;234:486-494.
- DeBowes LJ, Mosier D, Logan E, et al. Association of periodontal disease and histologic lesions in multiple organs from 45 dogs. J Vet Dent 1996;13:57-60.
- Paquette DW. The periodontal infection-systemic disease link: a review of the truth or myth. J Int Acad Periodontol 2002;4(3):101-9.
- Janket SJ, Baird A, Chuang S, et al. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol 2003;95:559-596.
Alessandro De Simoi
Med Vet, Dip. EVDC
Italy
Other articles in this issue
Share on social media